Abstract
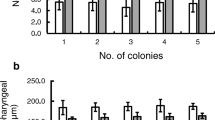
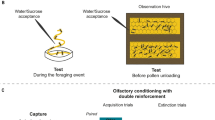
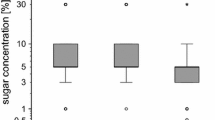
Honeybees harvest and use plant resins in a mixture called propolis to seal cracks and smooth surfaces in the nest architecture. Resins in the nest may be important in maintaining a healthy colony due to their antimicrobial properties. This study had two main objectives: (1) Provide initial insight on the learning capabilities of resin foraging honeybees; (2) analyze the sensitivity of resin foraging honeybees to tactile stimuli to elucidate its possible role as a mechanism behind resin foraging. The first objective provides insight into the phenotype of these bees as compared to other forager types, while the second creates a starting point for further work on behavioral mechanisms of resin foraging. Using tactile proboscis extension response conditioning, we found that resin foragers learned to associate two different tactile stimuli, the presence of a gap between two plates and a rough sandpaper surface, with a sucrose reward significantly better than pollen foragers. The results of differential tactile conditioning exhibited no significant difference in the ability of resin foragers to discriminate between smooth and rough surfaces as compared to pollen foragers. We also determined that the sucrose response thresholds (SRTs) of returning resin foragers were lower compared to returning pollen foragers, but both resin foragers and pollen foragers learned a floral odor equally well. This is the first study to examine SRTs and conditioning to tactile and olfactory stimuli with resin foraging honeybees. The results provide new information and identify areas for future research on resin collectors, an understudied foraging phenotype.




Similar content being viewed by others
References
Bastos EMAF, Simone M, Jorge DM, Soares AES, Spivak M (2008) In vitro study of the antimicrobial activity of Brazilian and Minnesota, USA propolis against Paenibacillus larvae. J Invertebr Pathol 97:273–281
Bollazzi M, Roces F (2007) To build or not to build: circulating dry air organizes collective building for climate control in the leaf-cutting ant Acromyrmex ambiguus. Anim Behav 74:1349–1355
Calderone NW, Johnson BR (2002) The within-nest behaviour of honeybee pollen foragers in colonies with a high or low need for pollen. Anim Behav 63:749–758
Crane E (1990) Bees and beekeeping. Cornell Univ, Press, Ithaca
de Brito SG, Ortigão-Farias JR, Gauthier M, Liu F, Giurfa M (2007) Taste perception in honeybees: just a taste of honey? Arthropod-Plant Inte 1:69–76
Downing HA (1992) Hole repair and the influence of learning on nest repair in the paper wasp, Polistes fuscatus (Hymenoptera: Vespidae). J Insect Behav 5:459–468
Downing HA (1994) Information analysis by the paper wasp, Polistes fuscatus, during nest construction (Hymenoptera, Vespidae). Insect Soc 41:361–377
Downing HA, Jeanne RL (1990) The regulation of complex behaviour in the paper wasp, Polistes fuscatus (Insecta, Hymenoptera, Vespidae). Anim Behav 39:105–124
Drezner-Levy T, Smith BH, Shafir S (2009) The effect of foraging specialization on various learning tasks in the honey bee (Apis mellifera). Behav Ecol Sociobiol 64:135–148
Erber J, Pribbenow B (2001) Antennal movements in the honeybee: How complex tasks are solved by a simple neuronal system. In: Cruse H et al (eds) Prerational intelligence: adaptive behavior and intelligent systems without symbols and logic, vol 1. Kluwer, Netherlands
Erber J, Kierzek S, Sander E, Grandy K (1998) Tactile learning in the honeybee. J Comp Physiol 228 A 183:737–744
Garedrew A, Lamprecht I, Schmolz E, Schricker B (2002) The varroacidal action of propolis: a laboratory assay. Apidologie 33:41–50
Goode K, Huber Z, Mesce KA, Spivak M (2006) Hygienic behavior of the honey bee (Apis mellifera) is independent of sucrose responsiveness and foraging ontogeny. Horm Behav 49:391–397
Giurfa M (2007) Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J Comp Physiol A 193:801–824
Hartz SM, Ben-Shahar Y, Tyler M (2001) Logistic growth curve analysis in associative learning data. Anim Cogn 4:185–189
Howse PE (1966) Air movement and termite behaviour. Nature 210:967–968
Johnson BR (2008) Global information sampling in the honeybee. Naturwissenschaften 95:523–530
Jones RJ (1980) Gallery construction by Nasutitermes costalis: polyethism and the behavior of individuals. Insect Soc 27:5–28
Kevan PG, Lane MA (1985) Flower petal microtexture is a tactile cue for bees. Proc Nat Acad Sci 82:4750–4752
Lee SH, Bardunias P, Yang RL (2008) Behavioral response of termites to tunnel surface irregularity. Behav Process 78:397–400
Masterman R, Smith BH, Spivak M (2000) Brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J Insect Behav 13:87–101
Masterman R, Ross R, Mesce K, Spivak M (2001) Olfactory and behavioral response thresholds to odors of diseased brood differ between hygienic and non-hygienic honey bees (Apis mellifera L.). J Comp Physiol A 187:441–452
Meyer W (1956) “Propolis bees” and their activities. Bee World 37:25–36
Nakamura J, Seeley TD (2006) The functional organization of resin work in honeybee colonies. Behav Ecol Sociobiol 60:339–349
Page RE Jr, Erber J (2001) Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften 89:91–106
Page RE Jr, Erber J, Fondrk MK (1998) The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 182:489–500
Pankiw T, Page RE Jr (1999) The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honeybees (Apis mellifera L.). J Comp Physiol A 185:207–213
Pankiw T, Waddington KD, Page Jr RE (2001) Modulation of sucrose response thresholds in honeybees (Apis mellifera L.): influence of genotype, feeding, and foraging experience
Roubik DW (1989) Ecology and natural history of tropical bees. Cambridge University Press, Cambridge
Roussel E, Carcaud J, Sandoz JC, Giurfa M (2009) Reappraising social insect behavior through aversive responsiveness and learning. PLoS ONE 4:e4197
Scheiner R, Erber J, Page RE Jr (1999) Tactile learning and the individual evaluation of the reward in honeybees (Apis mellifera L.). J Comp Physiol A 185:1–10
Scheiner R, Page RE Jr, Erber J (2001) The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honeybees (Apis mellifera L.). Neurobiol Learn Mem 76:138–150
Scheiner R, Barnert M, Erber J (2003) Variation in water and sucrose responsiveness during the foraging season affects proboscis extension learning in honey bees. Apidologie 34:67–72
Scheiner R, Page RE Jr, Erber J (2004) Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera). Apidologie 35:133–142
Scheiner R, Kuritz-Kaiser A, Menzel R, Erber J (2005) Sensory responsiveness and the effects of equal subjective rewards on tactile learning and memory of honeybees. Learn Mem 12:626–635
Seeley TD, Morse RA (1976) The nest of the honeybee (Apis mellifera L.). Insect Soc 23:495–512
Simone-Finstrom M, Spivak M (2010) Propolis and bee health: the natural history and significance of resin use by honey bees. Apidologie. doi:10.1051/apido/2010016
Simone M, Evans JD, Spivak M (2009) Resin collection and social immunity in honey bees. Evolution 63:3016–3022
Stuart AM (1967) Alarm, defense and construction behavior relationships in termites (Isoptera). Science 156:1123–1125
Acknowledgments
Funding was provided by a grant from the National Science Foundation (IOS-0717530) to M. Spivak and a National Science Foundation Graduate Research Fellowship awarded to M. Simone-Finstrom. We would also like to thank two anonymous reviewers for their thoughtful comments on this manuscript.
Ethical standards
The authors declare that the experiments comply with the current laws of the United States, where they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Giurfa
Rights and permissions
About this article
Cite this article
Simone-Finstrom, M., Gardner, J. & Spivak, M. Tactile learning in resin foraging honeybees. Behav Ecol Sociobiol 64, 1609–1617 (2010). https://doi.org/10.1007/s00265-010-0974-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0974-4