Abstract
Living in social groups facilitates cross-infection by parasites. However, empirical studies on indirect transmission within wildlife populations are scarce. We investigated whether asynchronous overnight refuge sharing among neighboring sleepy lizards, Tiliqua rugosa, facilitates indirect transmission of its ectoparasitic tick, Amblyomma limbatum. We fitted 18 neighboring lizards with GPS recorders, observed their overnight refuge use each night over 3 months, and counted their ticks every fortnight. We constructed a transmission network to estimate the cross-infection risk based on asynchronous refuge sharing frequencies among all lizards and the life history traits of the tick. Although self-infection was possible, the network provided a powerful predictor of measured tick loads. Highly connected lizards that frequently used their neighbors’ refuges were characterized by higher tick loads. Thus, indirect contact had a major influence on transmission pathways and parasite loads. Furthermore, lizards that used many different refuges had lower cross- and self-infection risks and lower tick loads than individuals that used relatively fewer refuges. Increasing the number of refuges used by a lizard may be an important defense mechanism against ectoparasite transmission in this species. Our study provides important empirical data to further understand how indirectly transmitted parasites move through host populations and influence individual parasite loads.
Similar content being viewed by others
Introduction
The costs of increased parasite transmission and infection have probably played an important role in the evolution of social organization because members of group-living species experience an increased total transmission risk compared to solitary individuals (Alexander 1974; Freeland 1976; Møller et al. 1993; Altizer et al. 2003; Nunn et al. 2004). However, some recent theoretical studies (Watve and Jog 1997; Wilson et al. 2003) have argued that association in social groups spatially separates the groups, thereby reducing between-group transmission, and this may compensate for the increased within-group transmission risk. Nevertheless, contact among members of a social group allows cross-infection by directly transmitted parasites. Contact frequencies among social group members are higher in large social groups, so directly transmitted parasites can be more abundant and prevalent in large groups (Cote and Poulin 1995). The adverse effects of parasites on hosts, such as reduced activity (Main and Bull 2000; Fenner and Bull 2008), home range size (Main and Bull 2000), or reproductive success (Arnold and Lichtenstein 1993; Møller 1993), should select for individual behavioral strategies that constrain social group size or reduce direct contact within groups. Behavior that directly reduces existing ectoparasite loads should also evolve within group-living species. For instance, allogrooming, a behavioral defense against ectoparasites, has been shown to occur in group-living species from insects to primates (Hughes et al. 2002; Zamma 2002; Radford and Du Plessis 2006).
Parasites that are not transmitted through direct host-to-host contact may benefit from spatial overlap in host home ranges. For instance, many parasites need to go through a period of development after leaving one host before they become infectious again. Parasites that spend this period in a host refuge will be indirectly transmitted among host individuals that share refuges asynchronously and may be more abundant and prevalent in local populations where sharing of refuges or sleeping sites is common (Butler and Roper 1996; Roper et al. 2002).
Opportunities for either direct or indirect parasite transmission will vary among dyads of host individuals as a function of the spatial and social organization of the host population. Network analysis provides a quantitative framework to determine these transmission heterogeneities (Krause et al. 2009) and to link them to individual pathogen infestation levels (Corner et al. 2003; Cross et al. 2004; Otterstatter and Thomson 2007; Naug 2008; Perkins et al. 2008; Godfrey et al. 2009). Directed networks, which contain information on asymmetrical interactions among host individuals, are of particular interest in the study of parasite transmission through asynchronous use of refuges. They incorporate the direction of transmission and describe possible transmission pathways through a population (Bell et al. 1999).
Here, we investigated indirect parasite transmission in a lizard population through spatial overlap and common use of overnight refuges. The parasitic ticks we studied detach in host refuges and molt before becoming infective to the next host. We developed a weighted directed network in the lizard population and used it to examine how dyadic connections through asynchronous refuge sharing may influence infection patterns by ticks. Due to the indirect transmission of the tick, we based the network on events when lizards used the same refuges at different times.
The host, the Australian sleepy lizard, Tiliqua rugosa, is a large (adults: snout-vent length ≥ 28 cm), long-lived (20 to 50 years) (Bull 1995) scincid lizard. It forms stable pair-bonds, with pair-partners in frequent social contact (Bull 1988; Leu et al. 2010). This may allow direct parasite transmission between pair-partners, while active individuals also avoid social contact with specific neighboring non-partner lizards, which may reduce their total direct transmission risk (Leu et al. 2010). For these ectothermic lizards, refuges are key resources for thermoregulation and for concealment when inactive. Sleepy lizards in our study area refuge under large bluebushes, Maireana sedifolia, or in mammal burrows (Kerr et al. 2003; Kerr and Bull 2004). They retain overlapping home ranges from year to year (Bull and Freake 1999) and choose non-randomly among a number of available refuges within home ranges (Kerr et al. 2003). Individuals repeatedly use the same set of overnight refuges and reuse large bushes more frequently than small ones (Kerr et al. 2003). Refuges can also be occupied by other individual lizards at different times.
In our study area, sleepy lizards are infected by the three-host tick Amblyomma limbatum. Larvae, nymphs, and adult ticks each feed on a new host (or reinfect the same host) (Smyth 1973). All life stages remain attached to host lizards for more than 2 weeks (Chilton 1989; Chilton and Bull 1991), and male ticks remain attached for many months while waiting for females to mate with (Andrews and Bull 1980). Engorged ticks normally detach at night in a host refuge. They molt to the next stage and then wait for their next host in that refuge (Petney et al. 1983; Chilton and Bull 1993b; Kerr and Bull 2006b). Remaining in lizard refuges increases tick exposure to new hosts and reduces risks of desiccation and predation by ants, the two major threats to off-host tick survival (Bull et al. 1988; Chilton and Bull 1993a, b). Ticks that detach outside of refuges are unlikely to survive to become infectious (Bull and Smyth 1973; Chilton and Bull 1993a).
Transmission of A. limbatum from one host to the next therefore relies on the two host lizards occupying the same overnight refuge asynchronously and within a time window of infection after a tick has detached from the first host. The beginning of that time window is set by how long a tick takes to molt and become ready to attach to a new host and the end by how long it can survive while waiting for a host.
Our aim was to determine whether transmission networks based on asynchronous refuge use could predict tick loads in the sleepy lizard. Although lizards may share refuges concurrently with their pair-partner or occasionally with extra-pair neighbors, this is not relevant for the transmission of the tick, which requires a time to elapse before it becomes infective. We constructed a directed weighted network that reflected the transmission pathway of the tick through a local lizard population. We calculated node in-strength as a measure of cross-infection risk. In our network, node in-strength measured how often a target lizard (the node) shared refuges within the time window of infection after other lizards had used the refuge. We predicted that individuals with higher in-strength would be more prone to parasitic infection, resulting in higher tick loads. We also included self-infection in our analysis, where a lizard re-used one of its own refuges within the time window of infection. We documented heterogeneous local refuge and population densities within our study group. High refuge densities and low population densities may result in an increased number of refuges used by an individual and a higher proportion of refuges that are used exclusively. An increased number of refuges, coupled with a higher proportion of exclusively used refuges, would result in lower refuge sharing and lower re-use probabilities. Therefore, we predicted that lizards using many different refuges would experience a lower infection risk.
Methods
Study site
We observed all resident sleepy lizards in a 700 × 1,000-m study site near Bundey Bore Station, in the mid-north of South Australia (33°54′16″ S, 139°20′43″ E). The area supports homogenous chenopod shrubland, dominated by bluebushes, M. sedifolia, which provide overnight lizard refuges (Kerr et al. 2003).
GPS tagging of study animals
In our study area, sleepy lizards are most active during spring and early summer (mid September to mid December) (Bull 1987; Firth and Belan 1998), at the time when we conducted our study. In August 2007, we captured 21 resident adult lizards (ten males, 11 females) that occupied adjacent home ranges in the study site. These lizards were part of a larger continuous population around the study site. We used surgical tape to attach a 37-g unit to the tail of each lizard, which included a data logger, a GPS device, and a radio transmitter. The unit weighed 4.9% of an average-sized lizard (750 g). After the study (mid December), we removed the units and released all lizards. We detected no skin damage or irritation where the units were attached and lizards naturally shed their skin in the following months. We believe the GPS devices did not adversely affect lizard behavior, because movement activity appeared similar to untagged lizards. Attachment of similar devices to this species has been widely used for several decades with no apparent adverse effect (Bull et al. 1998). For example, the mating behavior of tagged lizards has been regularly observed (How 2001; Kerr et al. 2004; Michniewicz 2004).
We could locate and individually recognize lizards by their unique radio transmitter frequency. Between 15 September and 15 December 2007, the data loggers recorded the GPS location of each lizard every 10 min if it had moved in that period. We synchronized the data recording process among all GPS devices, so that all locations were recorded at the same time. We recaptured each lizard once every fortnight to download the data and to replace the unit battery. At that time we recorded the number of attached ticks of each life stage. Since all life stages remain attached to host lizards for more than 14 days (Chilton 1989; Chilton and Bull 1991), we were confident that this survey interval allowed us to detect all ticks transmitted during the study period. Handling time, less than 60 min per fortnight, was excluded from the data set. We have previously used this method to record dyadic associations while lizards were away from their refuges and active and to describe their social network (Leu et al. 2010). Here, we used the same data set to develop a parasite transmission network.
Time window of infection
We considered adult ticks to be background infestation because they remain attached to host lizards for long periods, and we focused our analysis on the transmission of larvae and nymphs. We calculated the time window of infection based on previous reports of the time taken by larvae from detachment to molting and of the duration of survival of unfed nymphs after molting. Over 112 days at our study site, the mean daily maximal temperature under a typical bush was 30°C. Under similar conditions, the mean time for an engorged larva to molt to a nymph is 8 days (Chilton et al. 2000), and the mean time that unfed post-molt nymphs survive desiccation is 31 days (Chilton and Bull 1993a). We assumed for our model that there was a time window of infection from 9–39 days after a host had first used a refuge. As molting and survival times may vary under different climatic conditions, for different life stages, and under different levels of predation, our network with a 31-day window of infection represents one of several possible models that we could have used to predict empirical tick loads.
Overnight refuge sharing
We deduced the overnight refuge location of each lizard as the last GPS location record on each day. The last record marked the end of daily activity because the GPS devices did not record locations when lizards had been inactive. If a lizard remained inactive in the same refuge over consecutive days, the location was only recorded once. This reduced the number of days that the lizard was monitored over the study period. It occurred in 94 out of 1248 observations of lizards in overnight refuges. In the remaining 92.5% of cases, lizards stayed continuously in a refuge for one night. Thus, for our model, we assumed that all transmission events, i.e., detachment of engorged ticks and attachment of waiting unfed ticks, happened during the first night of refuge use. We based our potential transmission events solely on lizard movement, because unfed A. limbatum ticks do not actively move towards new hosts (Petney et al. 1983). For each lizard on each night, we calculated distances between its recorded refuge location on that night and the refuge location of each other lizard on each night within the time window of infection (i.e., on the following 9–39 days). Bushes used as refuges have an average canopy area of 4 m2 (Kerr et al. 2003), so we considered two locations within 2 m of each other to represent occupation of the same refuge. We applied the same distance criterion to other overnight refuges such as burrows. All GPS devices produced comparable average location records and had a median horizontal precision of 6 m (Leu et al. 2010). Hence, we considered that two lizards could have used the same refuge if their GPS recorded locations were up to 14 m apart. This is a conservative estimate that probably overestimates the level of refuge sharing and the opportunities for parasite transmission. Similarly, we calculated distances among all possible pairs of refuge locations of each lizard and used the same distance threshold to determine the number of different refuges each lizard used during the study.
Network construction
We constructed a weighted directed transmission network, based on asynchronous overnight refuge sharing events and the particular infection risk of each of these events. In the transmission network, we placed a directed edge from lizard A to lizard B if lizard A used a refuge and then lizard B used the same refuge in the subsequent period of day 9 to day 39. We calculated an edge weight to represent the transmission risk that lizard A (node of origin) posed on lizard B (node of destination). At each refuge use (or re-use), ticks could detach from lizard A and be waiting to attach to lizard B. So, the risk of lizard B becoming infected with ticks from lizard A through sharing a refuge once depended on how often lizard A had previously used this refuge (range 1–31 times, the duration of the time window of infection). The total risk to become infected by lizard A was the sum of all infection risks of each refuge sharing event, when lizard B followed lizard A in the use of a refuge. This was represented by the edge weight. Because of the asynchronous timing for transmission, the edge weights in opposite directions between two individuals (nodes) were asymmetrical.
Network analysis
We excluded three lizards from the analysis because they had low home range overlap with only a few lizards of the study group. These lizards probably shared space and refuges predominantly with other untagged lizards outside the study group. We would have underestimated node in-strength values for these lizards. We excluded these lizards from the analysis but not from the network construction or the derivative of network parameters for other lizards, so that their influence on the studied group of lizards was still taken into account.
Some general reviews have addressed sex differences in parasite prevalence and infection and transmission rates (Poulin 1996; Zuk and McKean 1996). In the sleepy lizard, tick infestation levels do not differ between host sexes (Bull and Burzacott 1993). Instead of intersexual differences, we investigated the effect of individual lizard behavior on the transmission dynamics within a local population. We focused our analysis on the more commonly detected tick life stages, larvae and nymphs (omitting adult ticks). These were also the stages for which we had derived the time window of infection. We used the median of the fortnightly counts of larvae plus nymphs as the dependent variable in our analyses. An important measurement for the analysis of transmission networks is node strength, sometimes also termed node degree in a weighted network (Naug 2008). Node strength incorporates the frequency of interactions relevant to transmission, as well as the number of individuals each individual interacted with. It is defined as the total weight of all edges connected to a node (Croft et al. 2008). We calculated node in-strength, which includes all edges towards the target lizard (node), i.e., in the direction of transmission. The node in-strength represented the total risk of an individual to become infected based on its own and its neighbors’ refuge sharing behavior. Hence, we predicted a positive correlation between node in-strength of individual lizards and their tick infestation levels. High values of in-strength can either result from many inward edges with moderate edge weight or from few edges with high edge weight. For each individual, we standardized the in-strength value by dividing by the number of overnight refuge records, that is, by the number of days each individual was monitored. This accounted for different “sample size” per individual. We termed the standardized in-strength the “cross-infection risk”. In order to put the cross-infection risk into perspective, we also calculated the risk of self-infection for each lizard, through re-use of its own refuges within the time window of infection. We calculated the self-infection risk similarly to the cross-infection risk and also standardized it by dividing by the number of days the individual was monitored.
We used Pearson correlation analysis if data met the assumption of normality. Otherwise we used Spearman rank correlation analysis. We calculated Spearman rank correlation coefficients to investigate whether parasite load was correlated with cross-infection risk or with self-infection risk, and we further analyzed whether the two infection methods differed in their strength. Using many different refuges may reduce the frequency of using previously occupied refuges. Hence, we investigated whether individuals that used more refuges experienced lower cross- and self-infection risks and whether this translated into a lower parasite load. We standardized the number of different refuges each lizard used by dividing by the number of days the individual was monitored. We used Pearson correlation coefficients to investigate whether the standardized number of different refuges was correlated with cross-infection risk or with self-infection risk. We further used Spearman rank correlation coefficient to investigate whether the standardized number of different refuges correlated with parasite load. Because network-derived measurements, such as strength, are relational non-independent data (Croft et al. 2008), we used randomization tests to estimate the probability that the observed test statistic was obtained by chance. Since our network was based on dyadic interactions (refuge sharing of dyads of lizards), we randomized node labels (parasite load, number of refuges) among nodes (lizards) and re-calculated the test statistic (James et al. 2009). We repeated this 1,000 times to achieve a consistent frequency distribution of the randomly generated test statistic values (Bejder et al. 1998). Following Croft et al. (2008), we calculated Monte Carlo P-values as the quotient of the number of times the randomly generated values exceeded or were below the observed value, depending on our hypothesis. For consistency, we also calculated Monte Carlo P-values for the measure of self-infection, although not technically a network-derived measurement. We used PopTools (Hood 2008) to analyze our transmission networks and NetDraw (Borgatti 2002) to illustrate them.
Results
Median tick load of larvae plus nymphs ranged from zero to seven (N = 18 lizards), zero to five for male lizards (N = 9), and zero to seven for female lizards (N = 9). Sexes did not differ in tick load (Mann–Whitney U = 33.0, P = 0.49, N = 18). We made 1,248 observations of lizards in overnight refuges, with 48–85 observations per lizard (mean = 69.3, SE = 2.5, N = 18). Individual lizards used a mean of 22 different refuge sites over the study period (range 12–39). Over the whole study, the 18 lizards used 229 different refuges with a mean distance to the nearest refuge site of 25.8 m (SE = 1.2). The number of times an individual came back to its most commonly used refuge ranged from 8 to 22 (mean = 13.2, SE = 1.1, N = 18). Occasionally, lizards remained inactive in an overnight refuge over consecutive days. In six cases, lizards stayed long enough to allow self-infection. These few cases were not included in the calculation of self-infection because refuge locations were only recorded once during periods of continuous inactivity, but we believe this had little overall effect.
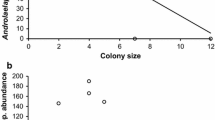
From the transmission network of the study population (Fig. 1) and correlation analyses of network parameters and infection risks (Table 1), we found that node in-strength (divided by the number of days monitored) was positively correlated with median tick load (Fig. 2). Thus, lizards that were exposed to high cross-infection risk also had high tick loads. Similarly, self-infection risk (divided by the number of days monitored) and median tick load were positively correlated (Fig. 3). Comparison of the cross- and self-infection risk showed no significant difference in their strength (paired t-test: t 17 = 1.64, Monte Carlo P = 0.068). Finally, the number of different refuges used was negatively correlated with node in-strength (both divided by the number of days monitored) (Fig. 4). Thus, lizards that used more refuges experienced a lower cross-infection risk. Similarly, the number of different refuges used was negatively correlated with self-infection risk (both divided by the number of days monitored) (Fig. 5). These reduced infection risks translated into a negative correlation between the number of different refuges used (divided by the number of days monitored) and median tick load (Fig. 6).
Discussion
Many reviews of social networks refer to their potential role in parasite and disease transmission (Bascompte 2007; Krause et al. 2007; Wey et al. 2008; Krause et al. 2009). This study contributes empirical data to support this assumption and shows the value of alternative networks, based on asynchronous sharing of refuges, in understanding how indirectly transmitted parasites move through a host population.
Parasite transmission opportunities varied among dyads of individuals, resulting from the spatial organization of the population. In the sleepy lizard, home ranges overlap among neighboring lizards (Kerr and Bull 2006a) and each individual uses a set of overnight refuges that can also be occupied by other lizards at different times. This behavior enables the transmission of the tick A. limbatum from one lizard to the next. Unfed ticks have low mobility and will not actively move towards new host individuals, instead relying on host movement for contact (Petney et al. 1983). We constructed a network based on asynchronous refuge sharing that reflected the tick transmission pathway through a local population. We used some simplifying assumptions in deriving the time window of infection and considered that all transmission activities took place on the first day of refuge use. Nevertheless, the transmission network provided a powerful predictor of tick loads.
Lizards that were highly connected in the network were more prone to parasitic infection, which was reflected by higher tick loads. This is consistent with other studies reporting a positive relationship between social network position of hosts and their infestation level with immobile parasites (Corner et al. 2003; Otterstatter and Thomson 2007; Godfrey et al. 2009). Our study differed from previous studies in deriving a transmission network from delayed transmission opportunities. In our study, high cross-infection risk, measured as standardized node in-strength, resulted from many refuge sharing events among neighbors with high transmission risks. Although repeated use of the same refuges also exposed lizards to potential self-infection, the model that only considered cross-infection risk was a good predictor of tick load. Lizards that frequently used the same refuges as their neighbors were exposed to high cross-infection risk and suffered higher tick loads.
Animals that re-use their own previously occupied locations, such as roost, nest, or refuge sites, are also exposed to the risk of self-infection. For example, Reckardt and Kerth (2007) showed that Bechstein’s bat, Myotis bechsteinii, avoids reusing roost locations when highly infective puparia of the bat fly Basilia nana are likely to be present. Our study showed that self-infection through repeated use of the same refuges seems to play a role in the transmission of the tick since there was a positive correlation between self-infection risk and tick load. Both cross- and self-infection risk arise from use of previously occupied refuges. Both risks were similarly correlated with parasite load, indicating that both infection types contribute to the total tick load.
Our results clearly suggest that indirect spatial interaction via asynchronously shared refuges increases the risk of pathogen and parasite transmission. We have previously shown that individuals in this population avoid social contact with specific non-partner neighbors while active (Leu et al. 2010) and this may be one way to reduce transmission risk. Another way to reduce infection risk may be to use multiple different refuges. Increasing the number of refuges used might increase the proportion of exclusively used refuges and decrease the probability of refuge sharing and re-use. While using more refuges to decrease the self-infection risk, individuals might not have increased their exposure to neighboring lizards and infective ticks in their refuges. The negative correlation between tick load and number of refuges used supports this view. However, the density of available refuge bushes is spatially variable (Kerr et al. 2003) and mammal burrows, which are used as alternative preferred refuges during periods of high summer temperatures, are scarce. Thus, although our study population occupied homogenous scrubland, availability of suitable refuges may be limited. There may be competition for high-quality areas determining the number of different refuges each lizard can occupy. Use of multiple refuges is a defense mechanism against ectoparasite transmission as also used by European badgers, Meles meles, and Brants’ whistling rats, Parotomys brantsii, where individuals that switch more frequently between sleeping chambers have lower parasite loads (Butler and Roper 1996; Roper et al. 2002). In those species, experimental reduction of parasite loads led to reduced switching between sleeping chambers (Butler and Roper 1996; Roper et al. 2002).
We argue that, in sleepy lizards, high node connectivity, shown by high in-strength values, resulted in high parasite load. An alternative interpretation of our results is that levels of network connectivity are the consequence of parasite load rather than the cause. That is, high parasite loads alter individual behavior to result in a more central network position. There is much experimental evidence that parasites and pathogens alter animal social behavior. In meerkats, Suricata suricatta, for example, experimental reduction of ectoparasites reduced overall grooming rates, and this influenced social behaviors, such as rates of unprompted submission behavior (Madden and Clutton-Brock 2009). In these cases, parasite load generally reduced the level of activity and social contact. Thus, we would expect that this reduces rather than increases the level of connectivity of infected individuals. Supporting this prediction, a previous study showed that high tick loads are associated with the separation of previously monogamous male–female sleepy lizard pairs, which were in frequent direct contact (Bull and Burzacott 2006). Another study showed that lizards with high tick loads move shorter distances in a day (Main and Bull 2000). This may result in using fewer different refuges which we have shown as correlated with high infection risks. Experimental manipulation of individual parasite infestation levels in a known network may provide further insight into cause and consequence. Nevertheless, here, we argue that in the sleepy lizard high node connectivity is more likely to lead to high tick loads. This is consistent with the report that sleepy lizards have increased tick loads after periods of high temperatures when multiple lizards repeatedly share the same cool temperature mammal burrows (Kerr and Bull 2006b). Because burrows are scarce, lizards are generally unable to avoid previously used burrows.
Although our evidence is correlational, it is consistent with the hypothesis that high contact frequencies result in high pathogen prevalence and abundance (Cote and Poulin 1995). We have shown that network analysis provides important information about potential pathways for parasite transmission in wildlife populations. More importantly, we have presented evidence that indirect contact, based on asynchronous resource sharing, can have a major influence on transmission pathways and parasite loads. This is of particular interest as it suggests that indirectly transmitted parasites may generate spatial structure in a population.
References
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383
Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, Dobson AP, Ezenwa V, Jones KE, Pedersen AB, Poss M, Pulliam JRC (2003) Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu Rev Ecol Evol Syst 34:517–547
Andrews RH, Bull CM (1980) Mating behaviour in the Australian reptile tick Aponomma hydrosauri. Anim Behav 28:1280–1286
Arnold W, Lichtenstein AV (1993) Ectoparasite loads decrease the fitness of alpine marmots (Marmota marmota) but are not a cost of sociality. Behav Ecol 4:36–39
Bascompte J (2007) Networks in ecology. Basic Appl Ecol 8:485–490
Bejder L, Fletcher D, Bräger S (1998) A method for testing association patterns of social animals. Anim Behav 56:719–725
Bell DC, Atkinson JS, Carlson JW (1999) Centrality measures for disease transmission networks. Soc Networks 21:1–21
Borgatti SP (2002) Netdraw network visualization. Analytic Technologies, Harvard, MA
Bull CM (1987) A population study of the viviparous Australian lizard, Trachydosaurus rugosus (Scincidae). Copeia 3:749–757
Bull CM (1988) Mate fidelity in an Australian lizard Trachydosaurus rugosus. Behav Ecol Sociobiol 23:45–49
Bull CM (1995) Population ecology of the sleepy lizard, Tiliqua rugosa, at Mt Mary, South Australia. Aust J Ecol 20:393–402
Bull CM, Smyth M (1973) The distribution of three species of reptile ticks, Aponomma hydrosauri Denny, Amblyomma albolimbatum Neumann, and Amb. limbatum Neumann. II. Water balance of nymphs and adults in relation to distribution. Aust J Zool 21:103–110
Bull CM, Burzacott D (1993) The impact of tick load on the fitness of their lizard hosts. Oecologia 96:415–419
Bull CM, Freake MJ (1999) Home-range fidelity in the Australian sleepy lizard, Tiliqua rugosa. Aust J Zool 47:125–132
Bull CM, Burzacott D (2006) The influence of parasites on the retention of long-term partnerships in the Australian sleepy lizard, Tiliqua rugosa. Oecologia 146:675–680
Bull CM, Chilton NB, Sharrad RD (1988) Risk of predation for two reptile tick species. Exp Appl Acarol 5:93–99
Bull CM, Cooper SJB, Baghurst BC (1998) Social monogamy and extra-pair fertilization in an Australian lizard, Tiliqua rugosa. Behav Ecol Sociobiol 44:63–72
Butler JM, Roper TJ (1996) Ectoparasites and sett use in European badgers. Anim Behav 52:621–629
Chilton NB (1989) Life cycle adaptations and their implications in the distribution of two parapatric species of tick. PhD thesis, Flinders University
Chilton NB, Bull CM (1991) A comparison of the reproductive parameters of females of two reptile tick species. Int J Parasitol 21:907–911
Chilton NB, Bull CM (1993a) A comparison of the off-host survival times of larvae and nymphs of two species of reptile ticks. Int J Parasitol 23:693–696
Chilton NB, Bull CM (1993b) Interspecific differences in microhabitat choice by two species of Australian reptile tick. Int J Parasitol 23:1045–1051
Chilton NB, Andrews RH, Bull CM (2000) Influence of temperature and relative humidity on the moulting success of Amblyomma limbatum and Aponomma hydrosauri (Acari: Ixodidae) larvae and nymphs. Int J Parasitol 30:973–979
Corner LAL, Pfeiffer DU, Morris RS (2003) Social-network analysis of Mycobacterium bovis transmission among captive brushtail possums (Trichosurus vulpecula). Prev Vet Med 59:147–167
Cote IM, Poulin R (1995) Parasitism and group size in social animals: a meta-analysis. Behav Ecol 6:159–165
Croft DP, James R, Krause J (2008) Exploring animal social networks. Princeton University Press, Princeton
Cross PC, Lloyd-Smith JO, Bowers JA, Hay CT, Hofmeyr M, Getz WM (2004) Integrating association data and disease dynamics in a social ungulate: bovine tuberculosis in African buffalo in the Kruger National Park. Ann Zool Fenn 41:879–892
Fenner A, Bull CM (2008) The impact of nematode parasites on the behaviour of an Australian lizard, the gidgee skink Egernia stokesii. Ecol Res 23:897–903
Firth BT, Belan I (1998) Daily and seasonal rhythms in selected body temperatures in the Australian lizard Tiliqua rugosa (Scincidae): field and laboratory observations. Physiol Zool 71:303–311
Freeland WJ (1976) Pathogens and the evolution of primate sociality. Biotropica 8:12–24
Godfrey S, Bull CM, James R, Murray K (2009) Network structure and parasite transmission in a group living lizard, the gidgee skink, Egernia stokesii. Behav Ecol Sociobiol 63:1045–1056
Hood GM (2008) PopTools version 3.0.6. URL http://www.cse.csiro.au/poptools
How TL (2001) Functions of monogamous pairing in the Australian skink, Tiliqua rugosa. PhD thesis, Flinders University
Hughes WOH, Eilenberg J, Boomsma JJ (2002) Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc R Soc B 269:1811–1819
James R, Croft DP, Krause J (2009) Potential banana skins in animal social network analysis. Behav Ecol Sociobiol 63:989–997
Kerr GD, Bull CM (2004) Field observations of extended locomotor activity at sub-optimal body temperatures in a diurnal heliothermic lizard (Tiliqua rugosa). J Zool 264:179–188
Kerr GD, Bull CM (2006a) Exclusive core areas in overlapping ranges of the sleepy lizard, Tiliqua rugosa. Behav Ecol 17:380–391
Kerr GD, Bull CM (2006b) Interactions between climate, host refuge use, and tick population dynamics. Parasitol Res 99:214–222
Kerr GD, Bull CM, Burzacott D (2003) Refuge sites used by the scincid lizard Tiliqua rugosa. Austral Ecol 28:152–160
Kerr GD, Bull CM, Cottrell GR (2004) Use of an ‘on board’ datalogger to determine lizard activity patterns, body temperature and microhabitat use for extended periods in the field. Wildl Res 31:171–176
Krause J, Croft DP, James R (2007) Social network theory in the behavioural sciences: potential applications. Behav Ecol Sociobiol 62:15–27
Krause J, Lusseau D, James R (2009) Animal social networks: an introduction. Behav Ecol Sociobiol 63:967–973
Leu ST, Bashford J, Kappeler PM, Bull CM (2010) Association networks reveal social organization in the sleepy lizard. Anim Behav 79:217–225
Madden JR, Clutton-Brock TH (2009) Manipulating grooming by decreasing ectoparasite load causes unpredicted changes in antagonism. Proc R Soc B 276:1263–1268
Main AR, Bull CM (2000) The impact of tick parasites on the behaviour of the lizard Tiliqua rugosa. Oecologia 122:574–581
Michniewicz RJ (2004) Pair fidelity in the Australian sleepy lizard, Tiliqua rugosa: a behavioural and genetic analysis. PhD thesis, Flinders University
Møller AP (1993) Ectoparasites increase the cost of reproduction in their hosts. J Anim Ecol 62:309–322
Møller AP, Dufva R, Allander K (1993) Parasites and the evolution of host social behavior. Adv Stud Behav 22:65–102
Naug D (2008) Structure of the social network and its influence on transmission dynamics in a honeybee colony. Behav Ecol Sociobiol 62:1719–1725
Nunn CL, Altizer S, Sechrest W, Jones KE, Barton RA, Gittleman JL (2004) Parasites and the evolutionary diversification of primate clades. Am Nat 164:S90–S103
Otterstatter M, Thomson J (2007) Contact networks and transmission of an intestinal pathogen in bumble bee (Bombus impatiens) colonies. Oecologia 154:411–421
Perkins SE, Ferrari MF, Hudson PJ (2008) The effects of social structure and sex-biased transmission on macroparasite infection. Parasitology 135:1561–1569
Petney TN, Andrews RH, Bull CM (1983) Movement and host finding by unfed nymphs of two Australian reptile ticks. Aust J Zool 31:717–721
Poulin R (1996) Sexual inequalities in helminth infections: a cost of being a male? Am Nat 147:287
Radford A, Du Plessis M (2006) Dual function of allopreening in the cooperatively breeding green woodhoopoe, Phoeniculus purpureus. Behav Ecol Sociobiol 61:221–230
Reckardt K, Kerth G (2007) Roost selection and roost switching of female Bechstein’s bats (Myotis bechsteinii) as a strategy of parasite avoidance. Oecologia 154:581–588
Roper TJ, Jackson TP, Conradt L, Bennett NC (2002) Burrow use and the influence of ectoparasites in Brants’ whistling rat Parotomys brantsii. Ethology 108:557–564
Smyth M (1973) The distribution of three species of reptile ticks, Aponomma hydrosauri (Denny), Amblyomma albolimbatum Neumann, and Amb. limbatum Neumann I. Distribution and hosts. Aust J Zool 21:91–101
Watve MG, Jog MM (1997) Epidemic diseases and host clustering: an optimum cluster size ensures maximum survival. J Theor Biol 184:165–169
Wey T, Blumstein DT, Shen W, Jordan F (2008) Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim Behav 75:333–344
Wilson K, Knell R, Boots M, Koch-Osborne J (2003) Group living and investment in immune defence: an interspecific analysis. J Anim Ecol 72:133–143
Zamma K (2002) Grooming site preferences determined by lice infection among Japanese macaques in Arashiyama. Primates 43:41–49
Zuk M, McKean KA (1996) Sex differences in parasite infections: patterns and processes. Int J Parasitol 26:1009–1024
Acknowledgments
The study was approved by the Flinders University Animal Welfare Committee (approval no. 478 E232) in compliance with the Australian Code of Practice for the use of animals for scientific purposes and conducted under the Department of Environment and Heritage Permit to Undertake Scientific Research (permit no A23436 15). All procedures carried out in this study conformed to the current laws of Australia. This research was supported by funds from the Australian Research Council and the Holsworth Wildlife Research Endowment. S.T.L. was funded by the German Academic Exchange Service. Three anonymous referees provided valuable feedback on the manuscript. We thank Ron and Leona Clark for allowing access to their land, Geoff Cottrell for maintaining the data loggers, and Dale Burzacott for logistical support at the field site.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Downes
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Leu, S.T., Kappeler, P.M. & Bull, C.M. Refuge sharing network predicts ectoparasite load in a lizard. Behav Ecol Sociobiol 64, 1495–1503 (2010). https://doi.org/10.1007/s00265-010-0964-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0964-6