Abstract
Senders and receivers influence dynamic characteristics of the signals used for mate attraction over different time scales. On a moment-to-moment basis, interactions among senders competing for a mate influence dynamic characteristics, whereas the preferences of receivers of the opposite gender exert an influence over evolutionary time. We observed and recorded the calling patterns of the bird-voiced treefrog Hyla avivoca to assess how the dynamic characters of calls vary during interactions among groups of males in a chorus. This question was also addressed using playback experiments with males. Playback experiments with females showed how changes in dynamic call properties are likely to affect male mating success. Frogs calling in pairs, groups, or in response to playbacks produced longer calls than did isolated males. During call overlap, males often increased the duration of the silent interval (gaps) between the pulses of their calls so that the pulses of the calls of two neighbors interdigitated. This change resulted in increased variability of pulse rate, a traditionally static acoustic property; however, males also produced high proportions of non-overlapped calls in which variability in pulse rate was low and had species-typical values. Females preferred long calls to short- and average-duration calls, and non-overlapped calls to overlapped calls. Given a choice between pairs of overlapped calls, females preferred pairs in which the proportion of overlap was low and pairs in which the pulses of such calls interdigitated completely. The observed patterns of vocal competition thus reflect the preferences of conspecific females, which have influenced the evolution of the calling behavior of H. avivoca.







Similar content being viewed by others
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Bee MA, Gerhardt HC (2001) Habituation as a mechanism of reduced aggression between neighboring territorial male bullfrogs, Rana catesbeiana. J Comp Psychol 115:68–82
Bourne GR, Collins AC, Hoder AM, McCarthy CL (2001) Vocal communication and reproductive behavior of the frog Colostethus bebeei in Guyana. J Herpetol 35:272–281
Bradbury J, Vehrencamp S (1998) Principles of animal communication. Sinauer, Sunderland, MA
Brush JS, Narins PM (1989) Chorus dynamics of a neotropical amphibian assemblage: comparison of computer simulation and natural behavior. Anim Behav 37:33–44
Dundee HA, Rossman DA (1989) The amphibians and reptiles of Louisiana (1996). Louisiana State University Press, Baton Rouge, pp 87–89
Gerhardt HC (1991) Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim Behav 42:615–635
Gerhardt HC (2005) Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution 59:395–408
Gerhardt HC, Huber F (2002) Acoustic communication in insects and frogs: common problems and diverse solutions. University of Chicago Press, Chicago
Gerhardt HC, Klump GM (1988) Masking of acoustic signals by the chorus background noise in the green tree frog: a limitation on mate choice. Anim Behav 36:1247–1249
Gerhardt HC, Dyson ML, Tanner SD (1996) Dynamic properties of the advertisement calls of gray treefrogs: patterns of variability and female choice. Behav Ecol 7:7–18
Grafe TU (2003) Synchronized interdigitated calling in the Kuvangu running frog, Kassina kuvangensis. Anim Behav 66:127–136
Greenfield MD (1994) Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Am Zool 34:605–615
Hill PSM (1998) Environmental and social influences on calling effort in the prairie mole cricket (Gryllotalpa major). Behav Ecol 9(1):101–108
Höbel G, Gerhardt CH (2003) reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea). Evolution 57:894–904
Kamo M, Ghirlanda S, Enquist M (2002) The evolution of signal form: effects of learned versus inherited recognition. Proc R Soc Lond B 269(1502):1765–1771
Kelson KI, Simpson GD, VanArsdale RB, Haraden CC, Lettis WR (1996) Multiple late Holocene earthquakes along the Reelfoot fault, central New Madrid seismic zone. J Geophys Res 101(B3):6151–6157
Klump GM, Gerhardt HC (1987) Use of non-arbitrary acoustic criteria in mate choice by female gray tree frogs. Nature (London) 326:286–288
Marshall VT, Humfeld SC, Bee MA (2003) Plasticity of aggressive signaling and its evolution in male spring peepers Pseudacris crucifer. Anim Behav 65:1223–1234
Marshall VT, Schwartz JJ, Gerhardt HC (2006) Effects of heterospecific call overlap on the phonotactic behaviour of grey treefrogs. Anim Behav 72(2):449–459
McGregor PK, Dabelsteen T (1996) Communication networks. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic communication in birds. Cornell University Press, Ithaca, NY
McGregor PK, Peake TM (2000) Communication networks: social environments for receiving and signalling behaviour. Acta Ethol 2:71–81
Mennill DJ, Ratcliffe LM, Boag PT (2002) Female eavesdropping on male song contest in songbirds. Science 296:873
Narins PM (1992) Evolution of anuran chorus behavior: neural and behavioral constrain. Am Nat 139:S90–S104
Narins PM, Hödl W, Grabul DS (2003) Bimodal signal requisite for agonistic behavior in a dart-poison frog, Epipedobates femoralis. PNAS 100:577–580
Oliveira RF, McGregor PK, Latruffe C (1998) Know thine enemy: fighting fish gather information from observing conspecific interactions. Proc R Soc Lond B 265:1045–1049
Passmore NI, Telford SR (1981) The effect of chorus organization on mate localization in the painted reed frog (Hyperolius marmoratus). Behav Ecol Sociobiol 9:291–293
Pfennig K (1998) The evolution of mate choice and the potential for conflicts between species and mate-quality recognition. Proc R Soc Lond B 265:1743–1748
Pfennig K, Simovich MA (2002) Differential selection to avoid hybridization in two toad species. Evolution 56(9):1840–1848
Rheinlaender J, Gerhardt HC, Yager D, Capranica RR (1979) Accuracy of phonotaxis in the green treefrog (Hyla cinerea). J Com Physiol 133:247–255
Schul J, Bush SL (2002) Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proc R Soc Lond B 269:1847–1852
Schwartz JJ (1987) The function of call alternation in anuran amphibians: a test of three hypotheses. Evolution 41:461–471
Schwartz JJ (1993) Male calling behavior, female discrimination and acoustic interference in the Neotropical treefrog Hyla microcephala under realistic acoustic conditions. Behav Ecol Sociobiol 32:401–414
Schwartz JJ, Gerhardt HC (1989) Spatially mediated release from auditory masking in an anuran amphibian. J Comp Physiol A 166:37–41
Schwartz JJ, Gerhardt HC (1995) Directionality of the auditory system and call pattern recognition during acoustic interference in the gray treefrog, Hyla versicolor. Audit Neurosci 1:195–206
Schwartz JJ, Marshall VT (2006) Forms of call overlap and their impact on advertisement call attractiveness to females of the gray treefrog, Hyla versicolor. Bioacoustics 16:39–56
Schwartz JJ, Wells KD (1985) Intra- and interespecific vocal behavior of the neotropical treefrog Hyla microcephala. Copeia 1985:27–38
Schwartz JJ, Ressel S, Bevier C (1995) Carbohydrate and calling: depletion of muscle glycogen and the chorusing dynamics of the neotropical frog Hyla microcephala. Behav Ecol Sociobiol 37:125–135
Schwartz JJ, Buchanan BW, Gerhardt HC (2002) Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting. Behav Ecol Sociobiol 53:9–19
Shaw KL, Herhlihy D (2000) Acoustic preference functions and song variability in the Hawaiian cricket Laupaula cerasina. Proc R Soc Lond B 267:577–584
Vehrencamp SL (2000) Handicap index and conventional signal elements of bird songs. In: Espmark Y, Amundsen T, Rosenquist G (eds) Animal signals: signaling and signal design in animal communication. Tapir Academic Press, Trodheim, Norway, pp 277–300
Welch AM, Semlitsch RD, Gerhardt HC (1998) Call duration as an indicator of genetic quality in male gray treefrogs. Science 280:1928–1930
Wells KD, Schwartz JJ (2006) The behavioral ecology of anuran communication. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Hearing and sound communication in amphibians. V 28. Springer, Berlin, pp 44–86
Wells KD, Taigen TL (1986) The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav Ecol Sociobiol 19:9–18
Wiley RH (2006) Signal detection and animal communication. Adv Study Behav 36:217–247
Wollerman L, Wiley RH (2001) Background noise from a natural chorus alters female discrimination of male calls in a neotropical frog. Anim Behav 63:15–22
Acknowledgment
We thank Adam Boyette, Drew Dittmer, and Zapic Martínez for assistance in collecting data and testing frogs in the field. Bob Jones, Kristy Wharton, and Ray Semlitsch facilitated locality data for the Mississippi, Louisiana, and Tennessee populations, respectively. Mario and Crystal Sánchez, and Kristy Wharton kindly provided housing (and moral support) in the field; George K. and S. Adams made the long hours in the field feel less harsh. Bob Jones and Jerome Ford kindly granted permission to carry out our research in Mississippi and Louisiana, respectively. Gerlinde Höbel and J. J. Schwartz, along with two anonymous reviewers greatly improved this manuscript. This work was supported by a National Science Foundation (Doctoral Dissertation Improvement Grant to Martínez-Rivera and IBN-0091993 to Gerhardt), the Public Health Service (DHHS R01 DC05760 to Gerhardt), and the City of Mayagüez Department of Education, Mayagüez, Puerto Rico (to Martínez-Rivera).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Christensen-Dalsgaard.
Electronic supplementary material
Below is the link to the electronic supplementary material.
S1 Advertisement call
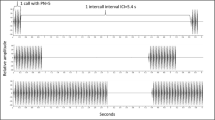
Sound file of average call of a male H. avivoca calling in a chorus. The call consist of 24 pulses of about 0.050 ms given at intervals of about 0.075 ms for a total call length of 3 s. Uninterrupted calls have a constant pulse rate of about 8.4 pulses per second at a temperature of about 24°C (see Fig. 1a and text) (WAV 423 KB)
S2 Call interaction
Stereo sound file of an advertisement-call interaction between two neighboring males. The first call, produced by male 1, is 2.34 s long and contains 18 pulses; the next call of male 1 is 2.46 s long and contains 17 pulses. The call is completely overlapped by the call of male 2, which is 3.17 s long and contains 24 pulses. During call overlap, both males increase the length of the inter-pulse interval, resulting in an irregular pulse rate during call overlap (see Fig. 1b for a graphic example of pulse interdigitation during call overlap) (WAV 1.76 MB)
Appendix 1
Appendix 1
Rights and permissions
About this article
Cite this article
Martínez-Rivera, C.C., Gerhardt, H.C. Advertisement-call modification, male competition, and female preference in the bird-voiced treefrog Hyla avivoca . Behav Ecol Sociobiol 63, 195–208 (2008). https://doi.org/10.1007/s00265-008-0650-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0650-0