Abstract
Purpose
The aim of this study is to examine the capacity of muscle tissue preserved on hamstring tendons forming candy-stripe grafts in order to improve tendon to bone ingrowth and ligamentization. We hypothesized that muscle tissue does possess a stem cell population that could enhance the healing process of the ACL graft when preserved on the tendons.
Methods
Human samples from gracilis and semitendinosus muscles were collected during ACL surgery from ten patients and from these tissue samples human muscle-derived stem cells and tendon-derived stem cells were isolated and propagated. Both stem cell populations were in-vitro differentiated into osteogenic lineage. Alkaline phosphatase activity was determined at days zero and 14 of the osteogenic induction and von Kossa staining to assess mineralization of the cultures. Total RNA was collected from osteoblast cultures and real time quantitative PCR was performed. Western-blot for osteocalcin and collagen type I followed protein isolation. Immunofluorescence double labeling of pericytes in muscle and tendon tissue was performed.
Results
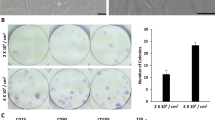
Mesenchymal stem cells from muscle and tendon tissue were isolated and expanded in cell culture. More time was needed to grow the tendon derived culture compared to muscle derived culture. Muscle derived stem cells exhibited more alkaline phosphatase actvity compared to tendon derived stem cells, whereas tendon derived stem cells formed more mineralized nodules after 14 days of osteoinduction. Muscle derived stem cells exhibited higher expression levels of bone sialoprotein, and tendon derived stem cells showed higher expression of dental-matrix-protein 1 and osteocalcin. Immunofluorescent staining against pericytes indicated that they are more abundant in muscle tissue.
Conclusions
These results indicate that muscle tissue is a better source of stem cells than tendon tissue. Achievement of this study is proof that there is vast innate capacity of muscle tissue for enhancement of bone-tendon integration and ligamentization of ACL hamstring grafts and consequently muscle tissue should not be treated as waste after harvesting.






Similar content being viewed by others
References
Zaffagnini S, Grassi A, Serra M, Marcacci M (2015) Return to sport after ACL reconstruction: how, when and why? A narrative review of current evidence. Joints 3(1):25–30
Robin BN, Jani SS, Marvil SC, Reid JB, Schillhammer CK, Lubowitz JH (2015) Advantages and disadvantages of transtibial, anteromedial portal, and outside-in femoral tunnel drilling in single-bundle anterior cruciate ligament reconstruction: a systematic review. Arthroscopy 31(7):1412–1417
Sherman OH, Banffy MB (2004) Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy 20(9):974–980
Carey JL, Dunn WR, Dahm DL, Zeger SL, Spindler KP (2009) A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg Am 91(9):2242–2250
Komiyama H, Arai Y, Kajikawa Y, Yoshida A, Morihara T, Terauchi R, Kida Y, Fujiwara H, Kawata M, Kubo T (2012) The fate and role of bone graft-derived cells after autologous tendon and bone transplantation into the bone tunnel. J Orthop Sci 18(6):994–1004
Scheffler SU, Unterhauser FN, Weiler A (2008) Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 16(9):834–842
Howell SM (2001) Autogenous graft choices in ACL reconstruction. Curr Opin Orthop 12:149–155
Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, Simic P, Bobinac D, Lah B, Legovic D, Vukicevic S (2004) Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med 32(7):1619–1625
Takigami J, Hashimoto Y, Yamasaki S, Terai S, Nakamura H (2015) Direct bone-to-bone integration between recombinant human bone morphogenetic protein-2-injected tendon graft and tunnel wall in an anterior cruciate ligament reconstruction model. Int Orthop 39(7):1441–1447
Schwarting T, Schenk D, Frink M, Benolken M, Steindor F, Oswald M, Ruchholtz S, Lechler P (2015) Stimulation with bone morphogenetic protein-2 (BMP-2) enhances bone-tendon integration in vitro. Connect Tissue Res 57(2):99–112
Bissell L, Tibrewal S, Sahni V, Khan WS (2014) Growth factors and platelet rich plasma in anterior cruciate ligament reconstruction. Curr Stem Cell Res Ther 10(1):19–25
Vogrin M, Rupreht M, Dinevski D, Haspl M, Kuhta M, Jevsek M, Knezevic M, Rozman P (2010) Effects of a platelet gel on early graft revascularization after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind, clinical trial. Eur Surg Res 45(2):77–85
Zhang X, Ma Y, Fu X, Liu Q, Shao Z, Dai L, Pi Y, Hu X, Zhang J, Duan X, Chen W, Chen P, Zhou C, Ao Y (2016) Runx2-modified adipose-derived stem cells promote tendon graft integration in anterior cruciate ligament reconstruction. Sci Rep 6:19073
Caplan AI (2009) New era of cell-based orthopedic therapies. Tissue Eng Part B Rev 15(2):195–200
Caplan AI (2015) Adult mesenchymal stem cells: when, where, and How. Stem Cells Int 2015:628767
Lavasani M, Lu A, Thompson SD, Robbins PD, Huard J, Niedernhofer LJ (2013) Isolation of muscle-derived stem/progenitor cells based on adhesion characteristics to collagen-coated surfaces. Methods Mol Biol 976:53–65
Matic I, Antunovic M, Brkic S, Josipovic P, Mihalic KC, Karlak I, Ivkovic A, Marijanovic I (2016) Expression of OCT-4 and SOX-2 in bone marrow-derived human mesenchymal stem cells during osteogenic differentiation. Open Access Maced J Med Sci 4(1):9–16
Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G (2005) Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev 19(9):1093–1104
Antunovic M, Kriznik B, Ulukaya E, Yilmaz VT, Mihalic KC, Madunic J, Marijanovic I (2015) Cytotoxic activity of novel palladium-based compounds on leukemia cell lines. Anticancer Drugs 26(2):180–186
Stevanovic V, Blagojevic Z, Petkovic A, Glisic M, Sopta J, Nikolic V, Milisavljevic M (2013) Semitendinosus tendon regeneration after anterior cruciate ligament reconstruction: can we use it twice? Int Orthop 37(12):2475–2481
Davies OG, Grover LM, Eisenstein N, Lewis MP, Liu Y (2015) Identifying the cellular mechanisms leading to heterotopic ossification. Calcif Tissue Int 97 (5):432–444
Sun L, Hou C, Wu B, Tian M, Zhou X (2013) Effect of muscle preserved on tendon graft on intra-articular healing in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 21(8):1862–1868
Unterhauser FN, Bail HJ, Hoher J, Haas NP, Weiler A (2003) Endoligamentous revascularization of an anterior cruciate ligament graft. Clin Orthop Relat Res 414:276–288
Kleiner JB (1986) Origin of replacement cels for the anterior cruciate ligament autograft. J Orthop Res 4(4):466–474
Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9(1):11–15
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3):301–313
Shino K (1984) Replacement of the anterior cruciate ligament by an allogenic tendon graft. An experimental study in the dog. J Bone Joint Surg (Br) 66(5):672–681
Kuroda R, Kurosaka M, Yoshiya S, Mizuno K (2000) Localization of growth factors in the reconstructed anterior cruciate ligament: immunohistological study in dogs. Knee Surg Sports Traumatol Arthrosc 8(2):120–126
Yoshikawa T, Tohyama H, Enomoto H, Matsumoto H, Toyama Y, Yasuda K (2006) Expression of vascular endothelial growth factor and angiogenesis in patellar tendon grafts in the early phase after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 14(9):804–810
Tohyama H, Yoshikawa T, Ju YJ, Yasuda K (2009) Revascularization in the tendon graft following anterior cruciate ligament reconstruction of the knee: its mechanisms and regulation. Chang Gung Med J 32(2):133–139
Weiler A, Forster C, Hunt P, Falk R, Jung T, Unterhauser FN, Bergmann V, Schmidmaier G, Haas NP (2004) The influence of locally applied platelet-derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med 32(4):881–891
Hunt P, Rehm O, Weiler A (2006) Soft tissue graft interference fit fixation: observations on graft insertion site healing and tunnel remodeling 2 years after ACL reconstruction in sheep. Knee Surg Sports Traumatol Arthrosc 14(12):1245–1251
Weiler A, Unterhauser FN, Bail HJ, Huning M, Haas NP (2002) Alpha-smooth muscle actin is expressed by fibroblastic cells of the ovine anterior cruciate ligament and its free tendon graft during remodeling. J Orthop Res 20(2):310–317
Dustmann M, Schmidt T, Gangey I, Unterhauser FN, Weiler A, Scheffler SU (2008) The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc 16(4):360–369
Thomopoulos S, Marquez JP, Weinberger B, Birman V, Genin GM (2006) Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech 39(10):1842–1851
Rothrauff BB, Tuan RS (2014) Cellular therapy in bone-tendon interface regeneration. Organogenesis 10(1):13–28
Kida Y, Morihara T, Matsuda K, Kajikawa Y, Tachiiri H, Iwata Y, Sawamura K, Yoshida A, Oshima Y, Ikeda T, Fujiwara H, Kawata M, Kubo T (2012) Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J Shoulder Elb Surg 22(2):197–205
Kinneberg KR, Galloway MT, Butler DL, Shearn JT (2013) The native cell population does not contribute to central-third graft healing at 6, 12, or 26 weeks in the rabbit patellar tendon. J Orthop Res 31(4):638–644
Bunker DL, Ilie V, Ilie V, Nicklin S (2014) Tendon to bone healing and its implications for surgery. Muscles Ligaments Tendons J 4(3):343–350
Oguma H, Murakami G, Takahashi-Iwanaga H, Aoki M, Ishii S (2001) Early anchoring collagen fibers at the bone-tendon interface are conducted by woven bone formation: light microscope and scanning electron microscope observation using a canine model. J Orthop Res 19(5):873–880
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
There is no funding source.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
None.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00264-017-3488-0.
Rights and permissions
About this article
Cite this article
Ćuti, T., Antunović, M., Marijanović, I. et al. Capacity of muscle derived stem cells and pericytes to promote tendon graft integration and ligamentization following anterior cruciate ligament reconstruction. International Orthopaedics (SICOT) 41, 1189–1198 (2017). https://doi.org/10.1007/s00264-017-3437-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3437-y