Abstract
Purpose
Articular cartilage has some capacity for self-repair. Clinically used low-intensity pulsed ultrasound (LIPUS) and pulsed electromagnetic field (PEMF) treatments were compared in their potency to prevent degeneration using an explant model of porcine cartilage.
Methods
Explants of porcine cartilage and human osteoarthritic cartilage were cultured for four weeks and subjected to daily LIPUS or PEMF treatments. At one, two, three and four weeks follow-up explants were prepared for histological assessment or gene expression (porcine only).
Results
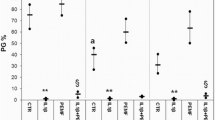
Non-treated porcine explants showed signs of atrophy of the superficial zone starting at one week. Treated explants did not. In LIPUS-treated explants cell clusters were observed. In PEMF-treated explants more hypertrophic-like changes were observed at later follow up. Newly synthesized tissue was present in treated explants. Gene expression profiles did indicate differences between the two methods. Both methods reduced expression of the aggrecan and collagen type II gene compared to the control. LIPUS treatment of human cartilage samples resulted in a reduction of degeneration according to Mankin scoring. PEMF treatment did not.
Conclusions
LIPUS or PEMF prevented degenerative changes in pig knee cartilage explants. LIPUS reduced degeneration in human cartilage samples. LIPUS treatment seems to have more potency in the treatment of osteoarthritis than PEMF treatment.






Similar content being viewed by others
References
Lafeber FP, Intema F, van Roermund PM, Marijnissen AC (2006) Unloading joints to treat osteoarthritis, including joint distraction. Curr Opin Rheumatol 18:519–525
Mastbergen SC, Saris DB, Lafeber FP (2013) Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol 9:277–290
Ploegmakers JJW, van Roermund PM, van Melkebeek J, Lammens J, Bijlsma JWJ, Lafeber FPJG, Marijnissen ACA (2005) Prolonged clinical benefit from joint distraction in the treatment of ankle osteoarthritis. Osteoarthr Cartil 13:582–588
Ivkovic A, Marijanovic I, Hudetz D, Porter RM, Pecina M, Evans CH (2011) Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed) 3:923–944
Haapala J, Arokoski JP, Ronkko S, Agren U, Kosma VM, Lohmander LS, Tammi M, Helminen HJ, Kiviranta I (2001) Decline after immobilisation and recovery after remobilisation of synovial fluid IL1, TIMP, and chondroitin sulphate levels in young beagle dogs. Ann Rheum Dis 60:55–60
Haapala J, Arokoski JPA, Hyttinen MM, Lammi M, Tammi M, Kovanen V, Helminen HJ, Kiviranta I (1999) Remobilization does not fully restore immobilization induced articular cartilage atrophy. Clin Orthop Rel Res 362:218–255
Cook SD, Salkeld SL, Popich-Patron LS, Ryaby JP, Jones DG, Barrack RL (2001) Improved cartilage repair after treatment with low-intensity pulsed ultrasound. Clin Orthop Rel Res 391S:231–243
Korstjens CM, van der Rijt RH, Albers GH, Semeins CM, Klein-Nulend J (2008) Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro. Med Biol Eng Comput 46:1263–1270
Min BH, Woo JI, Cho HS, Choi BH, Park SJ, Choi MJ, Park SR (2006) Effects of low-intensity ultrasound (LIUS) stimulation on human cartilage explants. Scand J Rheumatol 35:305–311
Zhang ZJ, Huckle J, Francomano CA, Spencer RGS (2002) The influence of pulsed low-intensity ultrasound on matrix production of chondrocytes at different stages of differentiation: An explant study. Ultrasound Med Biol 28:1547–1553
Zhang ZJ, Huckle J, Francomano CA, Spencer RGS (2003) The effects of pulsed low-intensity ultrasound on chondrocyte viability, proliferation, gene expression and matrix production. Ultrasound Med Biol 29:1645–1651
Aaron RK, Ciombor DM (1998) Therapeutic potential of electric fields in skeletal morphogenesis. In: Buckwalter JA, Ehrlich MG, Sandell LJ and Trippel SB (ed) Therapeutic potential of electric fields in skeletal morphogenesis. American Academy of Orthopaedic Surgeons, Rosemont, IL, pp 589–610
Benazzo F, Cadossi M, Cavani F, Fini M, Giavaresi G, Setti S, Cadossi R, Giardino R (2008) Cartilage repair with osteochondral autografts in sheep: effect of biophysical stimulation with pulsed electromagnetic fields. J Orthop Res 26:631–642
Boopalan PR, Arumugam S, Livingston A, Mohanty M, Chittaranjan S (2011) Pulsed electromagnetic field therapy results in healing of full thickness articular cartilage defect. Int Orthop 35:143–148
De Mattei M, Pasello M, Pellati A, Stabellini G, Massari L, Gemmati D, Caruso A (2003) Effects of electromagnetic fields on proteoglycan metabolism of bovine articular cartilage explants. Connect Tissue Res 44:154–159
Fini M, Giavaresi G, Carpi A, Nicolini A, Setti S, Giardino R (2005) Effects of pulsed electromagnetic fields on articular hyaline cartilage: review of experimental and clinical studies. Biomed Pharmacother 59:388–394
Ongaro A, Pellati A, Masieri FF et al (2011) Chondroprotective effects of pulsed electromagnetic fields on human cartilage explants. Bioelectromagnetics 32:543–551
Vincenzi F, Targa M, Corciulo C et al (2013) Pulsed electromagnetic fields increased the anti-inflammatory effect of A(2)A and A(3) adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS ONE 8:e65561
De Mattei M, Caruso A, Pezzetti F, Pellati A, Stabellini G, Sollazzo V, Traina GC (2001) Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect Tissue Res 42:269–279
De Mattei M, Pellati A, Pasello M, Ongaro A, Setti S, Massari L, Gemmati D, Caruso A (2004) Effects of physical stimulation with electromagnetic field and insulin growth factor-I treatment on proteoglycan synthesis of bovine articular cartilage. Osteoarthr Cartil OARS Osteoarthr Res Soc 12:793–800
Fioravanti A, Nerucci F, Collodel G, Markoll R, Marcolongo R (2002) Biochemical and morphological study of human articular chondrocytes cultivated in the presence of pulsed signal therapy. Ann Rheum Dis 61:1032–1033
Pezzetti F, De Mattei M, Caruso A, Cadossi R, Zucchini P, Carinci F, Traina GC, Sollazzo V (1999) Effects of pulsed electromagnetic fields on human chondrocytes: an in vitro study. Calcif Tissue Int 65:396–401
Sakai A, Suzuki K, Nakamura T, Norimura T, Tsuchiya T (1991) Effect of pulsing electromagnetic fields on cultured cartilage cells. Int Orthop 15:341–346
Schortinghuis J, Ruben JL, Raghoebar GM, Stegenga B (2004) Ultrasound to stimulate mandibular bone defect healing: a placebo-controlled single-blind study in rats. J Oral Maxillofac Surg 62:194–201
Choi BH, Woo JI, Min BH, Park SR (2006) Low-intensity ultrasound stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J Biomed Mater Res A 79:858–864
Bulstra SK, Drukker J, Kuijer R, Buurman WA, van der Linden AJ (1993) Thionin staining of paraffin and plastic embedded sections of cartilage. Biotech Histochem 68:20–28
Upton ML, Chen J, Guilak F, Setton LA (2003) Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res 21:963–969
Zou L, Zou X, Chen L, Li H, Mygind T, Kassem M, Bunger C (2008) Multilineage differentiation of porcine bone marrow stromal cells associated with specific gene expression pattern. J Orthop Res 26:56–64
Jung M, Gotterbarm T, Gruettgen A, Vilei SB, Breusch S, Richter W (2005) Molecular characterization of spontaneous and growth-factor-augmented chondrogenesis in periosteum-bone tissue transferred into a joint. Histochem Cell Biol 123:447–456
Chou CH, Cheng WT, Kuo TF, Sun JS, Lin FH, Tsai JC (2007) Fibrin glue mixed with gelatin/hyaluronic acid/chondroitin-6-sulfate tri-copolymer for articular cartilage tissue engineering: the results of real-time polymerase chain reaction. J Biomed Mater Res A 82:757–767
Lu L, Zhang Q, Pu LJ et al (2008) Dysregulation of matrix metalloproteinases and their tissue inhibitors is related to abnormality of left ventricular geometry and function in streptozotocin-induced diabetic minipigs. Int J Exp 89:125–137
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Aigner T, Stöss H, Weseloh G, Zeiler G, von der Mark K (1992) Activation of collagen type-II expression in osteoarthritic and rheumatoid cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol 62:337-345
Lotz MK, Otsuki S, Grogan SP, Sah R, Terkeltaub R, D’Lima D (2010) Cartilage cell clusters. Arthritis Rheum 62:2206–2218
Gobbi A, Lad D, Petrera M, Karnatzikos G (2014) Symptomatic early osteoarthritis of the knee treated with pulsed electromagnetic fields: two-year follow-up. Cartilage 5:78-85
McKibbin B, Maroudas A (1979) Nutrition and metabolism. In: Freeman MAR (ed) Nutrition and metabolism, 2nd edn. Pitman Medical, London, pp 461–486
O’Hara BP, Urban JPG, Maroudas A (1990) Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis 49:536–539
Pollack GH (2013) The Fourth Phase of Water. Beyond Solid, Liquid and Vapor. Ebner & Sons Publishers, Seattle, USA166
Zhao Q, Ovchinnikova K, Chai B, Yoo H, Magula J, Pollack GH (2009) Role of proton gradients in the mechanism of osmosis. J Phys Chem B 113:10708–10714
Zheng JM, Chin WC, Khijniak E, Khijniak E Jr, Pollack GH (2006) Surfaces and interfacial water: evidence that hydrophilic surfaces have long-range impact. Adv Colloid Interf Sci 127:19–27
Paukkonen K, Jurvelin J, Helminen HJ (1986) Effects of immobilization on the articular cartilage in young rabbits. A quantitative light microscopic stereological study. Clin Orthop Relat Res 206:270–280
Acknowledgments
Mathias Berg Johansen and Madelica Pituca are gratefully acknowledged for their experimental work. The Physiostim® apparatus was kindly provided by Evert Jan van de Kamp from Pro-Motion Medical bv Zwijndrecht, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, L., Ren, Y., van Kooten, T.G. et al. Low-intensity pulsed ultrasound (LIPUS) and pulsed electromagnetic field (PEMF) treatments affect degeneration of cultured articular cartilage explants. International Orthopaedics (SICOT) 39, 549–557 (2015). https://doi.org/10.1007/s00264-014-2542-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2542-4