Abstract
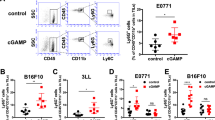
Stimulator of IFN genes (STING) spontaneously contributes to anti-tumor immunity by inducing type I interferons (IFNs) following sensing of tumor-derived genomic DNAs in the tumor-bearing host. Although direct injection of STING ligands such as cyclic diguanylate monophosphate (c-di-GMP) and cyclic [G(2′,5′)pA(3′,5′)p] (cGAMP) into the tumor microenvironment exerts anti-tumor effects through strong induction of type I IFNs and activation of innate and adaptive immunity, the precise events caused by STING in the tumor microenvironment remain to be elucidated. We describe here our finding that a CD45+ CD11bmid Ly6C+ cell subset transiently accumulated in mouse tumor microenvironment of 4T1 breast cancer, squamous cell carcinomas, CT26 colon cancer, or B16F10 melanoma tissue after intratumoral injection of cGAMP. The accumulated cells displayed a macrophage (M ) phenotype since the cells were positive for F4/80 and MHC class II and negative for Ly6G. Intratumoral cGAMP treatment did not induce Mφ accumulation in STING-deficient mice. Depletion of CD8+ T cell using anti-CD8 mAb impaired the anti-tumor effects of cGAMP treatment. Depletion of the Mφ using clodronate liposomes impaired the anti-tumor effects of cGAMP treatment. Functional analysis indicated that the STING-triggered tumor-migrating Mφ exhibited phagocytic activity, production of tumor necrosis factor alpha TNFα), and high expression levels of T cell-recruiting chemokines, Cxcl10 and Cxcl11, IFN-induced molecules, MX dynamin-like GTPase 1 (Mx1) and 2′-5′ oligoadenylate synthetase-like 1 (Oasl1), nitric oxide synthase 2 (Nos2), and interferon beta 1 (Ifnb1). These results indicate that the STING-triggered tumor-migrating Mφ participate in the anti-tumor effects of STING-activating compounds.





Similar content being viewed by others
Abbreviations
- c-di-GMP:
-
Cyclic diguanylate monophosphate
- cGAMP:
-
Cyclic [G(2′,5′)pA(3′,5′)p]
- DCs:
-
Dendritic cells
- IFNb1:
-
Interferon beta 1
- IFNγ:
-
Interferon gamma
- IFNs:
-
Interferons
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- MDSCs:
-
Myeloid-derived suppressor cells
- Mφ:
-
Macrophage(s)
- MO-MDSCs:
-
Monocytic MDSCs
- Mx1:
-
MX dynamin-like GTPase 1
- Nos2:
-
Nitric oxide synthase 2
- Oasl1:
-
2′-5′ Oligoadenylate synthetase-like 1
- PD-1:
-
Programmed death-1
- STING:
-
Stimulator of interferon genes
- TAM:
-
Tumor-associated Mφ
- TILs:
-
Tumor-infiltrating leukocytes
- TNFα:
-
Tumor necrosis factor alpha
References
Austyn JM, Gordon S (1981) F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11:805–815. doi:10.1002/eji.1830111013
Rose S, Misharin A, Perlman H (2012) A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A 81:343–350. doi:10.1002/cyto.a.22012
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164:6166–6173
Murray PJ, Allen JE, Biswas SK et al (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41:14–20. doi:10.1016/j.immuni.2014.06.008
Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI (2004) Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 172:989–999
Rodriguez PC, Quiceno DG, Zabaleta J et al (2004) Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 64:5839–5849. doi:10.1158/0008-5472.can-04-0465
Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A (2000) Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol 164:762–767
Torroella-Kouri M, Silvera R, Rodriguez D et al (2009) Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res 69:4800–4809. doi:10.1158/0008-5472.can-08-3427
Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791. doi:10.1126/science.1232458
Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE (2011) STING is a direct innate immune sensor of cyclic di-GMP. Nature 478:515–518. doi:10.1038/nature10429
Parvatiyar K, Zhang Z, Teles RM et al (2012) The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol 13:1155–1161. doi:10.1038/ni.2460
Ohkuri T, Ghosh A, Kosaka A, Zhu J, Ikeura M, David M, Watkins SC, Sarkar SN, Okada H (2014) STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer. Immunol Res 2:1199–1208. doi:10.1158/2326-6066.cir-14-0099
Woo SR, Fuertes MB, Corrales L et al (2014) STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41:830–842. doi:10.1016/j.immuni.2014.10.017
Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 166:678–689
Wakita D, Chamoto K, Ohkuri T et al (2009) IFN-gamma-dependent type 1 immunity is crucial for immunosurveillance against squamous cell carcinoma in a novel mouse carcinogenesis model. Carcinogenesis 30:1408–1415. doi:10.1093/carcin/bgp144
Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ (2013) Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell 51:226–235. doi:10.1016/j.molcel.2013.05.022
Yi G, Brendel VP, Shu C, Li P, Palanathan S, Cheng Kao C (2013) Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLoS One 8:e77846. doi:10.1371/journal.pone.0077846
Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gjewski TF (2011) Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med 208:2005–2016. doi:10.1084/jem.20101159
Parker KH, Beury DW, Ostrand-Rosenberg S (2015) Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res 128:95–139. doi:10.1016/bs.acr.2015.04.002
Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111:4233–4244. doi:10.1182/blood-2007-07-099226
Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE (2007) Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol 179:3926–3936
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35. doi:10.1038/nri978
Sanford DE, Belt BA, Panni RZ et al (2013) Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 19:3404–3415. doi:10.1158/1078-0432.ccr-13-0525
Fu J, Kanne DB, Leong M et al (2015) STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 7:283ra52. doi:10.1126/scitranslmed.aaa4306
Izumi K, Fang LY, Mizokami A, Namiki M, Li L, Lin WJ, Chang C (2013) Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol Med 5:1383–1401. doi:10.1002/emmm.201202367
Ruitenberg MJ, Vukovic J, Blomster L, Hall JM, Jung S, Filgueira L, McMenamin PG, Plant GW (2008) CX3CL1/fractalkine regulates branching and migration of monocyte-derived cells in the mouse olfactory epithelium. J Neuroimmunol 205:80–85. doi:10.1016/j.jneuroim.2008.09.010
Boyle ST, Faulkner JW, McColl SR, Kochetkova M (2015) The chemokine receptor CCR6 facilitates the onset of mammary neoplasia in the MMTV-PyMT mouse model via recruitment of tumor-promoting macrophages. Mol Cancer 14:115. doi:10.1186/s12943-015-0394-1
Minard-Colin V, Xiu Y, Poe JC, Horikawa M, Magro CM, Hamaguchi Y, Haas KM, Tedder TF (2008) Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcgammaRI, FcgammaRIII, and FcgammaRIV. Blood 112:1205–1213. doi:10.1182/blood-2008-01-135160
Tseng D, Volkmer JP, Willingham SB et al (2013) Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA 110:11103–11108. doi:10.1073/pnas.1305569110
Willingham SB, Volkmer JP, Gentles AJ et al (2012) The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA 109:6662–6667. doi:10.1073/pnas.1121623109
Kong F, Gao F, Li H et al (2016) CD47: a potential immunotherapy target for eliminating cancer cells. Clin Transl Oncol 18:1051–1055. doi:10.1007/s12094-016-1489-x
Deng L, Liang H, Xu M et al (2014) STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41:843–852. doi:10.1016/j.immuni.2014.10.019
Demaria O, De Gassart A, Coso S et al (2015) STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci USA 112:15408–15413. doi:10.1073/pnas.1512832112
Acknowledgements
The authors thank Mr. Hayakawa Toshiyuki and Ms. Hino Chihiro (at the Animal Laboratory for Medical Research, Center for Advanced Research and Education, Asahikawa Medical University) and Ms. Matsumoto Rie (at the Department of Pathology, Asahikawa Medical University) for devotedly maintaining the mice and Mr. Akutsu Hiroaki (at Center for Advanced Research and Education, Asahikawa Medical University) for technically supporting cell sorting.
This work was supported by grants from Japan Society for the Promotion of Science KAKENHI Grant Number 16K19070 (Ohkuri) and 16K19071 (Kosaka), the Akiyama Life Science Foundation (Ohkuri), and Innovative Research in Life Science from Asahikawa Medical University (Ohkuri and Kosaka).
Author contributions
Ohkuri and Kosaka preformed experiments, analyzed results, and made the figures; Ohkuri, Kosaka, Ishibashi, Kumai, Hirata, Ohara, Nagato, Oikawa, Aoki, Harabuchi, Celis, and Kobayashi discussed the results; Ohkuri, Kosaka, Celis, and Kobayashi designed the research and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ohkuri, T., Kosaka, A., Ishibashi, K. et al. Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol Immunother 66, 705–716 (2017). https://doi.org/10.1007/s00262-017-1975-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-1975-1