Abstract
Background
Anti-tumor vaccination is a new frontier in cancer treatment applicable to immunogenic neoplasms such as prostate and renal cancers. GX301 is a vaccine constituted by four telomerase peptides and two adjuvants, Montanide ISA-51 and Imiquimod.
Objective
The aim of this study was to analyze safety and tolerability of GX301 in an open-label, phase I/II trial. Immunological and clinical responses were also evaluated as secondary endpoints.
Experimental design
GX301 was administered by intradermally injecting 500 μg of each peptide (dissolved in Montanide ISA-51) in the skin of the abdomen. Imiquimod was applied as a cream at the injection sites. The protocol included 8 administrations at days 1, 3, 5, 7, 14, 21, 35, 63. Eligible patients were affected with stage IV prostate or renal cancer resistant to conventional treatments. Patients were clinically and immunologically monitored up to 6 months from the first immunization.
Results
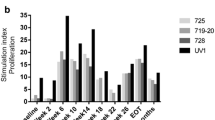
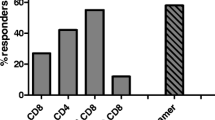
No grade 3–4 adverse events were observed. Evidence of vaccine-specific immunological responses was detected in 100 % of patients. Disease stabilization occurred in 4 patients. Prolonged progression-free survival and overall survival were observed in patients showing a full pattern of vaccine-specific immunological responses.
Conclusion
GX301 demonstrated to be safe and highly immunogenic. Further studies are needed to determine its clinical efficacy.


Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Rigamonti N, Bellone M (2012) Prostate cancer, tumor immunity and a renewed sense of optimism in immunotherapy. Cancer Immunol Immunother 61:453–468
Heidenreich A, Pfister D (2012) Treatment decisions for metastatic castration-resistant prostate cancer progressing after docetaxel chemotherapy: the role of cabazitaxel in the continuum of care. Eur Urol. doi:10.1016/j.eururo.2012.08.048
Figlin R, Sternberg C, Wood CG (2012) Novel agents and approaches for advanced renal cell carcinoma. J Urol 18:707–715
Kwek SS, Cha E, Fong L (2012) Unmasking the immune recognition of prostate cancer with CTLA4 blockade. Nat Rev Cancer 12:289–297
Perut F, Cenni E, Unger RE, Kirkpatrick CJ, Giunti A, Baldini N (2009) Immunogenic properties of renal cell carcinoma and the pathogenesis of osteolytic bone metastases. Int J Oncol 34:1387–1393
Gerritsen WR (2012) The evolving role of immunotherapy in prostate cancer. Ann Oncol 23(Supplement 8):viii22–viii27. doi:10.1093/annonc/mds259
Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H (2012) Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med doi. doi:10.1038/nm.2883
Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, Urdal DL (2012) Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. (Epub ahead of print) PMID: 22865266
Blackburn EH (1991) Structure and function of telomeres. Nature 350:569–573
Greider CW (1994) Mammalian telomere dynamics: healing, fragmentation shortening and stabilization. Curr Opin Genet Dev 4:203–211
Nugent CI, Lundblad V (1998) The telomerase reverse transcriptase: components and regulation. Genes Dev 12:1073–1185
Gomez DE, Armando RG, Farina HG, Menna PL, Cerrudo CS, Ghiringhelli PD, Alonso DF (2012) Telomere structure and telomerase in health and disease (review). Int J Oncol 41:1561–1569
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2013
Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA (1999) Creation of human tumour cells with defined genetic elements. Nature 400:464–468
Dhaene K, Van Marck E, Parwaresch R (2000) Telomeres, telomerase and cancer: an up-date. Virchows Arch 437:1–16
Vonderheide RH, Hahn WC, Schultze JL, Nadler LM (1999) The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity 10:673–679
Minev B, Hipp J, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M (2000) Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci 97:4796–4801
Filaci G, Fravega M, Setti M, Traverso P, Millo E, Fenoglio D, Negrini S, Ferrera F, Romagnoli AR, Basso M, Contini P, Rizzi M, Ghio M, Benatti U, Damonte G, Ravetti JL, Carmignani G, Zanetti M, Indiveri F (2006) Frequency of telomerase-specific CD8 + T lymphocytes in patients with cancer. Blood 107:1505–1512
Beatty GL, Vonderheide RH (2008) Telomerase as a universal tumor antigen for cancer vaccines. Expert Rev Vaccines 7:881–887
Brunsvig PF, Kyte JA, Kersten C, Sundstrøm S, Møller M, Nyakas M, Hansen GL, Gaudernack G, Aamdal S (2011) Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res 17:6847–6857
Hunger RE, Kernland Lang K, Markowski CJ, Trachsel S, Møller M, Eriksen JA, Rasmussen AM, Braathen LR, Gaudernack G (2011) Vaccination of patients with cutaneous melanoma with telomerase-specific peptides. Cancer Immunol Immunother 60:1553–1564
Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, Horger MS, Maksimovic O, Stenzl A, Hoerr I, Rammensee HG, Holderried TA, Kanz L, Pascolo S, Brossart P (2011) Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther 19:990–999
Berntsen A, Trepiakas R, Wenandy L, Geertsen PF, thor Straten P, Andersen MH, Pedersen AE, Claesson MH, Lorentzen T, Johansen JS, Svane IM (2008) Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: a clinical phase 1/2 trial. J Immunother 31:771–780
Cortez-Gonzalez X, Zanetti M (2007) Telomerase immunity from bench to bedside: round one. J Transl Med 5:12
Brunsvig PF, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S, Møller M, Eriksen JA, Gaudernack G (2006) Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother 55:1553–1564
Parkhurst MR, Riley JP, Igarashi T, Li Y, Robbins PF, Rosenberg SA (2004) hTERT: 540–548 peptide induces peptide-reactive T lymphocytes that do not recognize tumors endogenously expressing telomerase. Clin Cancer Res 10:4688–4698
Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, Coleman D, Niedzwiecki D, Gilboa E, Vieweg J (2003) Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res 63:2127–2133
Wenandy L, Sørensen RB, Sengeløv L, Svane IM, thor Straten P, Andersen MH (2008) The immunogenicity of the hTERT540-548 peptide in cancer. Clin Cancer Res 14:4–7
Schroers R, Huang XF, Hammer J, Zhang J, Chen SY (2002) Identification of HLA DR7-restricted epitopes from human telomerase reverse transcriptase recognized by CD4+ T-Helper cells. Cancer Res 62:2600–2605
Schroers R, Shen L, Rollins L, Rooney CM, Slawin K, Sonderstrup G, Huang XF, Chen SY (2003) Human telomerase reverse transcriptase-specific T-helper responses induced by promiscuous major histocompatibility complex class II-restricted epitopes. Clin Cancer Res 9:4743–4755
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Durando P, Fenoglio D, Boschini A, Ansaldi F, Icardi G, Sticchi L, Renzoni A, Fabbri P, Ferrera A, Parodi A, Bruzzone B, Gabutti G, Podda A, Del Giudice G, Fragapane E, Indiveri F, Crovari P, Gasparini R (2008) Safety and immunogenicity of two influenza virus subunit vaccines, with or without MF59 adjuvant, administered to human immunodeficiency virus type 1-seropositive and -seronegative adults. Clin Vaccine Immunol 15:253–259
Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M (2007) Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA 104:8947–8952
Godoy-Ramirez K, Mäkitalo B, Thorstensson R, Sandström E, Biberfeld G, Gaines H (2005) A novel assay for assessment of HIV-specific cytotoxicity by multiparameter flow cytometry. Cytometry A 68:71–80
Young NT, Mulder A, Cerundolo V, Claas FH, Welsh KI (1998) Expression of HLA class I antigens in transporter associated with antigen processing (TAP)-deficient mutant cell lines. Tissue Antigens 52:368–373
Czerkinsky C, Andersson G, Ekre HP, Nilsson LA, Klareskog L, Ouchterlony O (1988) Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods 110:29–36
Amyes E, McMichael AJ, Callan MF (2005) Human CD4+ T cells are predominantly distributed among six phenotypically and functionally distinct subsets. J Immunol 175:5765–5773
Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N (2007) Four functionally distinct populations of human effector-memory CD8 + T lymphocytes. J Immunol 178:4112–4119
Oh WK, Manola J, Babcic V, Harnam N, Kantoff PW (2006) Response to second-line chemotherapy in patients with hormone refractory prostate cancer receiving two sequences of mitoxantrone and taxanes. Urology 6:1235–1240
Michels J, Montemurro T, Murray N, Kollmannsberger C, Nguyen Chi K (2006) First- and second-line chemotherapy with docetaxel or mitoxantrone in patients with hormone-refractory prostate cancer: does sequence matter? Cancer 106:1041–1046
Parmiani G, Castelli C, Rivoltini L, Casati C, Tully GA, Novellino L, Patuzzo A, Tosi D, Anichini A, Santinami M (2003) Immunotherapy of melanoma. Semin Cancer Biol 13:391–400
Pardoll DM, Topalian SL (1998) The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol 10:588–594
Fernandez-Viña MA, Falco M, Sun Y, Stastny P (1992) DNA typing for HLA class I alleles: I. Subsets of HLA-A2 and of -A28. Hum Immunol 33:163–173
Ayyoub M, Migliaccio M, Guillaume P, Liénard D, Cerottini JC, Romero P, Lévy F, Speiser DE, Valmori D (2001) Lack of tumor recognition by hTERT peptide 540–548-specific CD8(+) T cells from melanoma patients reveals inefficient antigen processing. Eur J Immunol 31:2642–2651
Lev A, Denkberg G, Cohen CJ, Tzukerman M, Skorecki KL, Chames P, Hoogenboom HR, Reiter Y (2002) Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res 62:3184–3194
Vonderheide RH, Domchek SM, Schultze JL, George DJ, Hoar KM, Chen DY, Stephans KF, Masutomi K, Loda M, Xia Z, Anderson KS, Hahn WC, Nadler LM (2004) Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res 10:828–839
Dupont J, Latouche JB, Ma C, Sadelain M (2005) Artificial antigen-presenting cells transduced with telomerase efficiently expand epitope-specific, human leukocyte antigen-restricted cytotoxic T cells. Cancer Res 65:5417–5427
Moser M, Leo O (2010) Key concepts in immunology. Vaccine 28(Suppl 3):C2–C13
Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V (2002) Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines 1:111–118
Johnston D, Bystryn JC (2006) Topical imiquimod is a potent adjuvant to a weakly-immunogenic protein prototype vaccine. Vaccine 24:1958–1965
Church SE, Jensen SM, Twitty CG, Bahjat K, Hu HM, Urba WJ, Fox BA (2011) Multiple vaccinations: friend or foe. Cancer J 17:379–396
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Acknowledgments
This study was supported by grants from Genovax s.r.l. and Compagnia di San Paolo, Torino. Neither of the two sponsors of the study had any role in the study design, data collection, analysis, data interpretation, or writing of the manuscript.
Conflict of interest
GX301 is patented by Genovax s.r.l. Domenico Criscuolo is the President of Genovax; Gilberto Filaci, Francesco Indiveri, and Daniela Fenoglio are the stockholders of Genovax, while Francesco Boccardo is the member of the advisory board of Genovax; however, they worked at the project as academics so that they did not receive any payment by Genovax. All the other authors do not have any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Daniela Fenoglio and Paolo Traverso contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fenoglio, D., Traverso, P., Parodi, A. et al. A multi-peptide, dual-adjuvant telomerase vaccine (GX301) is highly immunogenic in patients with prostate and renal cancer. Cancer Immunol Immunother 62, 1041–1052 (2013). https://doi.org/10.1007/s00262-013-1415-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1415-9