Abstract
Purpose
Immunotherapy targeting disialoganglioside GD2 emerges as an important treatment option for neuroblastoma, a pediatric malignancy characterized by poor outcome. Here, we report the induction of a GD2-specific immune response with ganglidiomab, a new anti-idiotype antibody to anti-GD2 antibodies of the 14.18 family.
Experimental design and results
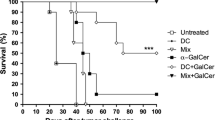
Ganglidiomab was generated following immunization of Balb/c mice with 14G2a, and splenocytes were harvested to generate hybridoma cells. Clones were screened by ELISA for mouse antibody binding to hu14.18. One positive clone was selected to purify and characterize the secreted IgG protein (κ, IgG1). This antibody bound to anti-GD2 antibodies 14G2a, ch14.18/CHO, hu14.18, and to immunocytokines ch14.18-IL2 and hu14.18-IL2 as well as to NK-92 cells expressing scFv(ch14.18)-zeta receptor. Binding of these anti-GD2 antibodies to the nominal antigen GD2 as well as GD2-specific lysis of neuroblastoma cells by NK-92-scFv(ch14.18)-zeta cells was competitively inhibited by ganglidiomab, proving GD2 surrogate function and anti-idiotype characteristics. The dissociation constants of ganglidiomab from anti-GD2 antibodies ranged from 10.8 ± 5.01 to 53.5 ± 1.92 nM as determined by Biacore analyses. The sequences of framework and complementarity-determining regions of ganglidiomab were identified. Finally, we demonstrated induction of a GD2-specific humoral immune response after vaccination of mice with ganglidiomab effective in mediating GD2-specific killing of neuroblastoma cells.
Conclusion
We generated and characterized a novel anti-idiotype antibody ganglidiomab and demonstrated activity against neuroblastoma.





Similar content being viewed by others
References
Yang RK, Sondel PM (2010) Anti-GD2 strategy in the treatment of neuroblastoma. Drugs Future 35:665
Modak S, Cheung NK (2007) Disialoganglioside directed immunotherapy of neuroblastoma. Cancer Invest 25:67–77
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX et al (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363:1324–1334
Zeng Y, Fest S, Kunert R, Katinger H, Pistoia V, Michon J et al (2005) Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol Immunol 42:1311–1319
Sen G, Chakraborty M, Foon KA, Reisfeld RA, Bhattacharya-Chatterjee MB (1998) Induction of IgG antibodies by an anti-idiotype antibody mimicking disialoganglioside GD2. J Immunother 21:75–83
Bhattacharya-Chatterjee M, Chatterjee SK, Foon KA (2002) Anti-idiotype antibody vaccine therapy for cancer. Expert Opin Biol Ther 2:869–881
Becker R, Eichler MK, Jennemann R, Bertalanffy H (2002) Phase I clinical trial on adjuvant active immunotherapy of human gliomas with GD2-conjugate. Br J Neurosurg 16:269–275
Chapman PB, Morrisey D, Panageas KS, Williams L, Lewis JJ, Israel RJ et al (2000) Vaccination with a bivalent G(M2) and G(D2) ganglioside conjugate vaccine: a trial comparing doses of G(D2)-keyhole limpet hemocyanin. Clin Cancer Res 6:4658–4662
Fest S, Huebener N, Weixler S, Bleeke M, Zeng Y, Strandsby A et al (2006) Characterization of GD2 peptide mimotope DNA vaccines effective against spontaneous neuroblastoma metastases. Cancer Res 66:10567–10575
Basak S, Birebent B, Purev E, Somasundaram R, Maruyama H, Zaloudik J et al (2003) Induction of cellular immunity by anti-idiotypic antibodies mimicking GD2 ganglioside. Cancer Immunol Immunother 52:145–154
Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD et al (2012) NK cells engineered to express a GD(2)-specific antigen receptor display built-in ADCC-like activity against tumor cells of neuroectodermal origin. J Cell Mol Med 16:569–581
Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA (1997) Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst 89:1586–1594
Wang Z, Raifu M, Howard M, Smith L, Hansen D, Goldsby R et al (2000) Universal PCR amplification of mouse immunoglobulin gene variable regions: the design of degenerate primers and an assessment of the effect of DNA polymerase 3′ to 5′ exonuclease activity. J Immunol Methods 233:167–177
Larrick JW, Danielsson L, Brenner CA, Abrahamson M, Fry KE, Borrebaeck CA (1989) Rapid cloning of rearranged immunoglobulin genes from human hybridoma cells using mixed primers and the polymerase chain reaction. Biochem Biophys Res Commun 160:1250–1256
Coloma MJ, Hastings A, Wims LA, Morrison SL (1992) Novel vectors for the expression of antibody molecules using variable regions generated by polymerase chain reaction. J Immunol Methods 152:89–104
Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A (2001) Calcein-acetoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol 8:1131–1135
Huebener H, Fest S, Strandsby A, Michalsky E, Preissner R, Zeng Y et al (2008) A rationally designed tyrosine hydroxylase DNA vaccine induces specific anti-neuroblastoma immunity. Mol Cancer Ther 7:2241–2251
Zeytin HE, Tripathi PK, Bhattacharya-Chatterjee M, Foon KA, Chatterjee SK (2000) Construction and characterization of DNA vaccines encoding the single-chain variable fragment of the anti-idiotype antibody 1A7 mimicking the tumor-associated antigen disialoganglioside GD2. Cancer Gene Ther 7:1426–1436
Hevey R, Ling CC (2012) Recent advances in developing synthetic carbohydrate-based vaccines for cancer immunotherapies. Future Med Chem 4:545–584
Foon KA, Sen G, Hutchins L, Kashala OL, Baral R, Banerjee M et al (1998) Antibody responses in melanoma patients immunized with an anti-idiotype antibody mimicking disialoganglioside GD2. Clin Cancer Res 4:1117–1124
Sen G, Chakraborty M, Foon KA, Reisfeld RA, Bhattacharya-Chatterjee MB (1998) Induction of IgG antibodies by an anti-idiotype antibody mimicking disialoganglioside GD2. J Immunother 21:75–83
Batova A, Strother DR, Castleberry RP, Eskenazi EE, Yu A (2002) Immune responses to an anti-idiotype monoclonal antibody 1A7 as a tumor vaccine in children with high risk neuroblastoma. AACR Proc 43 (Abstract)
Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC et al (2010) Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol 28:4969–4975
Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD et al (2011) Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118:6050–6056
Acknowledgments
We would like to thank Dr. M. Bhattacharya-Chatterjee, University of Kentucky, and Prof. Dr. R. Handgretinger, Center for Pediatric and Adolescence Medicine, University of Tübingen, for providing 1A7 and ch14.18-delta-CH2 for this study. Clinical grade ch14.18/CHO and hu14.18-IL2 were provided by Polymun, Vienna, Austria, and by Apeiron Biologics, Vienna, Austria, respectively. We also thank Prof. Dr. Winfried S. Wels and his group at the Georg-Speyer-Haus, Frankfurt am Main, Germany, for providing the NK-92 cells expressing the scFv(ch14.18)-zeta receptor. We also would like to acknowledge the cooperative group of pediatric oncologists of the SIOPEN-R-NET for the production of ch14.18/CHO antibody carried out by Polymun Scientific, Vienna, Austria, which was financed by charities throughout Europe. We also thank Andrea Plath and Theodor Koepp (University Medicine Greifswald, Pediatric Hematology and Oncology, Greifswald, Germany) for excellent technical assistance. Financial support was provided by the German Federal Ministry of Education and Research (BMBF, NGFNplus, ENGINE, and the Hector Stiftung) (Holger N. Lode), by a Kind-Philipp-Stiftung training grant (Diana Seidel), and by BMBF (FKZ03Z2CN12) within the ZIK-HIKE project (Sven Brandt and Hans-Peter Mueller).
Conflict of interest
There is no conflict of interest to be disclosed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lode, H.N., Schmidt, M., Seidel, D. et al. Vaccination with anti-idiotype antibody ganglidiomab mediates a GD2-specific anti-neuroblastoma immune response. Cancer Immunol Immunother 62, 999–1010 (2013). https://doi.org/10.1007/s00262-013-1413-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1413-y