Abstract
Purpose
To explore whole-lesion histogram analysis of the hepatobiliary phase (HBP) defect in indeterminate hypovascular liver lesions for predicting progression to arterial-enhancing hepatocellular carcinoma (HCC).
Methods
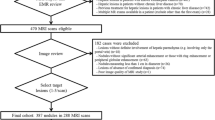
Twenty patients undergoing gadoxetic acid-enhanced MRI for HCC screening with 12° and 25° flip angle (FA) HBP acquisitions demonstrating an indeterminate lesion showing HBP hypointensity but no arterial enhancement were included. Volumes-of-interest were placed on HBP defects, from which histogram metrics were obtained. Associations between these metrics and progression to arterial-enhancing HCC on follow-up imaging were investigated. Lesions were also assessed for the presence of a signal abnormality on conventional sequences.
Results
40% of lesions progressed to arterial-enhancing HCC; 60% were stable at ≥6 months follow-up. Neither T2-hyperintensity increased diffusion signal nor portal/equilibrium phase washout was different between progressing and nonprogressing lesions (p = 1.0). Among direct signal intensity-based measures (overall mean; mean of bottom 10th, 10–25th, and 25–50th percentiles), area-under-the-curve (AUC) for prediction of progression to arterial-enhancing HCC was consistently higher at 25° (range 0.619–0.657) than at 12° (range 0.512–0.548). However, at both FAs, the four measures with highest AUC were measures related to lesion texture and heterogeneity [standard deviation (SD), coefficient of variation (CV), skewness, and entropy], having AUC of 0.655–0.750 at 12° and 0.686–0.800 at 25. The metric with highest AUC at 12° was SD (AUC = 0.750) and at 25° was CV (AUC = 0.800).
Conclusion
Whole-lesion histogram HBP measures of indeterminate hypovascular liver lesions may help predict progression to arterial-enhancing HCC by reflecting greater lesion heterogeneity, particularly at higher FA. Larger studies are therefore warranted.


Similar content being viewed by others
References
Wald C, Russo MW, Heimbach JK, et al. (2013) New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology 266(2):376–382
Golfieri R, Grazioli L, Orlando E, et al. (2012) Which is the best MRI marker of malignancy for atypical cirrhotic nodules: hypointensity in hepatobiliary phase alone or combined with other features? Classification after Gd-EOB-DTPA administration. J Magn Reson Imaging 36(3):648–657
Motosugi U, Bannas P, Sano K, Reeder SB (2015) Hepatobiliary MR contrast agents in hypovascular hepatocellular carcinoma. J Magn Reson Imaging 41(2):251–265
Takayasu K, Arii S, Sakamoto M, et al. (2013) Clinical implication of hypovascular hepatocellular carcinoma studied in 4474 patients with solitary tumour equal or less than 3 cm. Liver Int 33(5):762–770
Jang KM, Kim SH, Kim YK, Choi D (2015) Imaging features of subcentimeter hypointense nodules on gadoxetic acid-enhanced hepatobiliary phase MR imaging that progress to hypervascular hepatocellular carcinoma in patients with chronic liver disease. Acta Radiol 56(5):526–535
Motosugi U, Ichikawa T, Sano K, et al. (2011) Outcome of hypovascular hepatic nodules revealing no gadoxetic acid uptake in patients with chronic liver disease. J Magn Reson Imaging 34(1):88–94
Kim YK, Lee WJ, Park MJ, et al. (2012) Hypovascular hypointense nodules on hepatobiliary phase gadoxetic acid-enhanced MR images in patients with cirrhosis: potential of DW imaging in predicting progression to hypervascular HCC. Radiology 265(1):104–114
Willatt JM, Hussain HK, Adusumilli S, Marrero JA (2008) MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology 247(2):311–330
Krinsky GA, Lee VS, Theise ND, et al. (2001) Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology 219(2):445–454
Hyodo T, Murakami T, Imai Y, et al. (2013) Hypovascular nodules in patients with chronic liver disease: risk factors for development of hypervascular hepatocellular carcinoma. Radiology 266(2):480–490
Haradome H, Grazioli L, Al manea K, et al. (2012) Gadoxetic acid disodium-enhanced hepatocyte phase MRI: can increasing the flip angle improve focal liver lesion detection? J Magn Reson Imaging 35(1):132–139
Bashir MR, Husarik DB, Ziemlewicz TJ, et al. (2012) Liver MRI in the hepatocyte phase with gadolinium-EOB-DTPA: does increasing the flip angle improve conspicuity and detection rate of hypointense lesions? J Magn Reson Imaging 35(3):611–616
Lee ES, Lee JM, Yu MH, et al. (2014) High spatial resolution, respiratory-gated, t1-weighted magnetic resonance imaging of the liver and the biliary tract during the hepatobiliary phase of gadoxetic Acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr 38(3):360–366
Kumada T, Toyoda H, Tada T, et al. (2011) Evolution of hypointense hepatocellular nodules observed only in the hepatobiliary phase of gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol 197(1):58–63
Rosenkrantz AB, Obele C, Rusinek H, et al. (2015) Whole-lesion diffusion metrics for assessment of bladder cancer aggressiveness. Abdom Imaging 40(2):327–332
Kierans AS, Rusinek H, Lee A, et al. (2014) Textural differences in apparent diffusion coefficient between low- and high-stage clear cell renal cell carcinoma. AJR Am J Roentgenol 203(6):W637–W644
Donati OF, Mazaheri Y, Afaq A, et al. (2014) Prostate cancer aggressiveness: assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology 271(1):143–152
Frydrychowicz A, Lubner MG, Brown JJ, et al. (2012) Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging 35(3):492–511
Feuerlein S, Boll DT, Gupta RT, et al. (2011) Gadoxetate disodium-enhanced hepatic MRI: dose-dependent contrast dynamics of hepatic parenchyma and portal vein. AJR Am J Roentgenol 196(1):W18–W24
Prince MR, Chenevert TL, Foo TK, et al. (1997) Contrast-enhanced abdominal MR angiography: optimization of imaging delay time by automating the detection of contrast material arrival in the aorta. Radiology 203(1):109–114
Foo TK, Saranathan M, Prince MR, Chenevert TL (1997) Automated detection of bolus arrival and initiation of data acquisition in fast, three-dimensional, gadolinium-enhanced MR angiography. Radiology 203(1):275–280
Riederer SJ, Fain SB, Kruger DG, Busse RF (1999) Real-time imaging and triggering of 3D contrast-enhanced MR angiograms using MR fluoroscopy. MAGMA 8(3):196–206
Sharma P, Kalb B, Kitajima HD, et al. (2011) Optimization of single injection liver arterial phase gadolinium enhanced MRI using bolus track real-time imaging. J Magn Reson Imaging 33(1):110–118
Song M, Cho HJ, Cho YK, et al. (2013) Detecting hepatocellular carcinoma in gadoxetic-acid-enhanced hepatobiliary-phase MR imaging at 3T: comparing high and low flip angle. Jpn J Radiol 31(12):803–811
Royston P (1992) Which measures of skewness and kurtosis are best? Stat Med 11(3):333–343
Baek HJ, Kim HS, Kim N, Choi YJ, Kim YJ (2012) Percent change of perfusion skewness and kurtosis: a potential imaging biomarker for early treatment response in patients with newly diagnosed glioblastomas. Radiology 264(3):834–843
Rosenkrantz AB (2013) Histogram-based apparent diffusion coefficient analysis: an emerging tool for cervical cancer characterization? AJR Am J Roentgenol 200(2):311–313
Kierans AS, Bennett GL, Mussi TC, et al. (2013) Characterization of malignancy of adnexal lesions using ADC entropy: comparison with mean ADC and qualitative DWI assessment. J Magn Reson Imaging 37(1):164–171
Chong Y, Kim JH, Lee HY, et al. (2014) Quantitative CT variables enabling response prediction in neoadjuvant therapy with EGFR-TKIs: are they different from those in neoadjuvant concurrent chemoradiotherapy? PLoS One 9(2):e88598
Rosenkrantz AB, Triolo MJ, Melamed J, et al. (2015) Whole-lesion apparent diffusion coefficient metrics as a marker of percentage Gleason 4 component within Gleason 7 prostate cancer at radical prostatectomy. J Magn Reson Imaging 41(3):708–714
Chou CT, Chen YL, Su WW, Wu HK, Chen RC (2010) Characterization of cirrhotic nodules with gadoxetic acid-enhanced magnetic resonance imaging: the efficacy of hepatocyte-phase imaging. J Magn Reson Imaging 32(4):895–902
Lee MH, Kim SH, Park MJ, Park CK, Rhim H (2011) Gadoxetic acid-enhanced hepatobiliary phase MRI and high-b-value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. AJR Am J Roentgenol 197(5):W868–W875
Nakamura Y, Tashiro H, Nambu J, et al. (2013) Detectability of hepatocellular carcinoma by gadoxetate disodium-enhanced hepatic MRI: tumor-by-tumor analysis in explant livers. J Magn Reson Imaging 37(3):684–691
Choi JW, Lee JM, Kim SJ, et al. (2013) Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR images and their value as an imaging biomarker. Radiology 267(3):776–786
Kitao A, Matsui O, Yoneda N, et al. (2012) Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology 265(3):780–789
Matsuda M, Tsuda T, Yoshioka S, et al. (2014) Incidence for progression of hypervascular HCC in hypovascular hepatic nodules showing hyperintensity on gadoxetic acid-enhanced hepatobiliary phase in patients with chronic liver diseases. Jpn J Radiol 32(7):405–413
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors: No disclosures related to the work under consideration or outside of the submitted work.
Rights and permissions
About this article
Cite this article
Rosenkrantz, A.B., Pinnamaneni, N., Kierans, A.S. et al. Hypovascular hepatic nodules at gadoxetic acid-enhanced MRI: whole-lesion hepatobiliary phase histogram metrics for prediction of progression to arterial-enhancing hepatocellular carcinoma. Abdom Radiol 41, 63–70 (2016). https://doi.org/10.1007/s00261-015-0610-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0610-x