Abstract
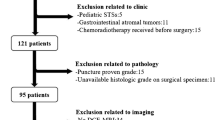
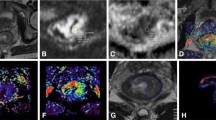
Purpose: To evaluate the role of computed tomography (CT) texture analysis in assessing response of soft tissue sarcoma (STS) treated with neoadjuvant bevacizumab (BVZ) plus radiotherapy in comparison to tumor size, density, and perfusion. Methods: In the phase II clinical trial, 20 patients with STSs received BVZ alone for 2 weeks followed by BVZ plus radiotherapy for 6 weeks prior to surgery. All patients received CT perfusion at baseline, 2 and 8 weeks after the therapy, and tumor blood flow (BF) was measured. In contrast enhanced CT image at the arterial peak enhancement time, mean of positive pixels (MPP) was measured as a texture parameter using texture analysis software, and tumor size and density were also measured. The percent changes of these parameters were compared with pathological response on surgical specimen. Results: After 2 weeks of the therapy, MPP and BF decreased by 10.42% and 20.08%, while changes of tumor size and density were not obvious. After 8 weeks, MPP, BF, and density decreased by 29.2% (p = 0.03), 53.2% (p = 0.001), and 30.41% (p = 0.005), respectively, without a significant change in size. The percent change of MPP after 8 weeks had a significant correlation with tumor necrosis in surgical specimen (r = −0.801, p < 0.001), whereas those of size, density, and BF did not. The receiver-operating characteristic analysis demonstrated that the percent change of MPP < −35.36% was an optimal cut-off value to differentiate pathological responders. Conclusion: The change of MPP is the best biomarker for the treatment response in STS.





Similar content being viewed by others
References
Clark MA, Fisher C, Judson I, Thomas JM (2005) Soft-tissue sarcomas in adults. N Engl J Med 353:701–711
Robinson E, Bleakney RR, Ferguson PC, O’Sullivan B (2008) Oncodiagnosis panel: 2007: multidisciplinary management of soft-tissue sarcoma. Radiographics 28:2069–2086
Eilber FC, Rosen G, Eckardt J, et al. (2001) Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol 19:3203–3209
Eisenhauer EA, Therasse P, Bogaerts J, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Goh V, Ganeshan B, Nathan P, et al. (2011) Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261:165–171
Jiang T, Kambadakone A, Kulkarni NM, Zhu AX, Sahani DV (2012) Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST). Invest Radiol 47:11–17
Wang X, Jacobs MA, Fayad L (2011) Therapeutic response in musculoskeletal soft tissue sarcomas: evaluation by MRI. NMR Biomed 24:750–763
Evilevitch V, Weber WA, Tap WD, et al. (2008) Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res 14:715–720
Ganeshan B, Miles KA, Young RC, Chatwin CR (2007) In search of biologic correlates for liver texture on portal-phase CT. Acad Radiol 14:1058–1068
Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V (2013) Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 266:177–184
Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K (2012) Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol 67:157–164
Ganeshan B, Abaleke S, Young RC, Chatwin CR, Miles KA (2010) Texture analysis of non-small cell lung cancer on unenhanced computed tomography: initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 10:137–143
Davnall F, Yip CS, Ljungqvist G, et al. (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589
Yoon SS, Duda DG, Karl DL, et al. (2011) Phase II study of neoadjuvant bevacizumab and radiotherapy for resectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys 81:1081–1090
Kambadakone AR, Sharma A, Catalano OA, Hahn PF, Sahani DV (2011) Protocol modifications for CT perfusion (CTp) examinations of abdomen-pelvic tumors: impact on radiation dose and data processing time. Eur Radiol 21:1293–1300
Hayano K, Shuto K, Koda K, et al. (2009) Quantitative measurement of blood flow using perfusion CT for assessing clinicopathologic features and prognosis in patients with rectal cancer. Dis Colon Rectum 52:1624–1629
Goh V, Halligan S, Wellsted DM, Bartram CI (2009) Can perfusion CT assessment of primary colorectal adenocarcinoma blood flow at staging predict for subsequent metastatic disease? A pilot study. Eur Radiol 19:79–89
Ganeshan B, Miles KA, Young RC, Chatwin CR (2009) Texture analysis in non-contrast enhanced CT: impact of malignancy on texture in apparently disease-free areas of the liver. Eur J Radiol 70:101–110
Ganeshan B, Burnand K, Young R, et al. (2011) Dynamic contrast-enhanced texture analysis of the liver initial assessment in colorectal cancer. Invest Radiol 46:160–168
Miles KA, Ganeshan B, Hayball MP (2013) CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 13:400–406
Miles KA, Ganeshan B, Rodriguez-Justo M, et al. (2014) Multifunctional imaging signature for V-KI-RAS2 Kirsten rat sarcoma Viral oncogene homolog (KRAS) mutations in colorectal cancer. J Nucl Med 55:386–391
Ganeshan B, Goh V, Mandeville HC, et al. (2013) Non–small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 266:326–336
Kim CK, Lim JH, Park CK, et al. (2005) Neoangiogenesis and sinusoidal capillarization in hepatocellular carcinoma: correlation between dynamic CT and density of tumor microvessels. Radiology 237:529–534
Shah D, Borys D, Martinez SR, et al. (2012) Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res 32:3911–3916
Benz MR, Czernin J, Allen-Auerbach MS, et al. (2009) FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res 15:2856–2863
Stacchiotti S, Collini P, Messina A, et al. (2009) High-grade soft-tissue sarcomas: tumor response assessment—pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology 251:447–456
Zhang H, Graham CM, Elci O, et al. (2013) Locally advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated with induction chemotherapy. Radiology 269:801–809
Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A (2007) CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology 244:486–493
Reiner CS, Roessle M, Thiesler T, et al. (2013) Computed tomography perfusion imaging of renal cell carcinoma. Invest Radiol 48:183–191
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22:796–802
Assignies GD, Couvelard A, Bahrami S, et al. (2009) Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology 250:407–416
West CC, Brown NJ, Mangham DC, Grimer RJ, Reed MW (2005) Microvessel density does not predict outcome in high grade soft tissue sarcoma. Eur J Surg Oncol. 31:1198–1205
Ravanelli M, Farina D, Morassi M, et al. (2013) Texture analysis of advanced non-small cell lung cancer (NSCLC) on contrast-enhanced computed tomography: prediction of the response to the first-line chemotherapy. Eur Radiol 23:3450–3455
Yip C, Landau D, Kozarski R, et al. (2014) Primary esophageal cancer: heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology 270:141–148
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, F., Hayano, K., Kambadakone, A.R. et al. Response assessment to neoadjuvant therapy in soft tissue sarcomas: using CT texture analysis in comparison to tumor size, density, and perfusion. Abdom Imaging 40, 1705–1712 (2015). https://doi.org/10.1007/s00261-014-0318-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0318-3