Abstract
Purpose
The aim of this study was to synthesize and preclinically evaluate an 18F-PSMA positron emission tomography (PET) tracer. Prostate-specific membrane antigen (PSMA) specificity, biodistribution, and dosimetry in healthy and tumor-bearing mice were determined.
Methods
Several conditions for the labeling of 18F-PSMA-11 via 18F-AlF-complexation were screened to study the influence of reaction temperature, peptide amount, ethanol volume, and reaction time. After synthesis optimization, biodistribution and dosimetry studies were performed in C57BL6 mice. For proof of PSMA-specificity, mice were implanted with PSMA-negative (PC3) and PSMA-positive (LNCaP) tumors in contralateral flanks. Static and dynamic microPET/computed tomography (CT) imaging was performed.
Results
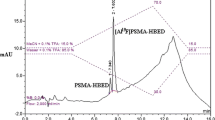
Quantitative labeling yields could be achieved with >97 % radiochemical purity. The 18F-PSMA-11 uptake was more than 24-fold higher in PSMA-high LNCaP than in PSMA-low PC3 tumors (18.4 ± 3.3 %ID/g and 0.795 ± 0.260 %ID/g, respectively; p < 4.2e-5). Results were confirmed by ex vivo gamma counter analysis of tissues after the last imaging time point. The highest absorbed dose was reported for the kidneys. The maximum effective dose for an administered activity of 200 MBq was 1.72 mSv.
Conclusion
18F-PSMA-11 using direct labeling of chelate-attached peptide with aluminum-fluoride detected PSMA-expressing tumors with high tumor-to-liver ratios. The kidneys were the dose-limiting organs. Even by applying the most stringent dosimetric calculations, injected activities of up to 0.56 GBq are feasible.




Similar content being viewed by others
References
Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–39. doi:10.1002/jcb.10661.
Eder M, Schafer M, Bauder-Wust U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–97. doi:10.1021/bc200279b.
Weineisen M, Schottelius M, Simecek J, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA targeted theranostic concept and first proof of concept human studies. J Nucl Med. 2015. doi:10.2967/jnumed.115.158550.
Mease RC, Foss CA, Pomper MG. PET imaging in prostate cancer: focus on prostate-specific membrane antigen. Curr Top Med Chem. 2013;13:951–62.
Schiavina R, Ceci F, Romagnoli D, et al. Ga-PSMA-PET/CT-guided salvage retroperitoneal lymph node dissection for disease relapse after radical prostatectomy for prostate cancer. Clin Genitourin Cancer. 2015. doi:10.1016/j.clgc.2015.06.004.
Budaus L, Leyh-Bannurah SR, Salomon G, et al. Initial experience of Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2015. doi:10.1016/j.eururo.2015.06.010.
Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–90. doi:10.2967/jnumed.115.160382.
Jadvar H. PSMA PET, in prostate cancer. J Nucl Med. 2015;56:1131–2. doi:10.2967/jnumed.115.157339.
Ceci F, Uprimny C, Nilica B, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–94. doi:10.1007/s00259-015-3078-6.
Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74. doi:10.2967/jnumed.115.154153.
Kabasakal L, Demirci E, Ocak M, et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl Med Commun. 2015;36:582–7. doi:10.1097/MNM.0000000000000290.
Chakraborty PS, Kumar R, Tripathi M, Das CJ, Bal C. Detection of brain metastasis with 68Ga-labeled PSMA ligand PET/CT: a novel radiotracer for imaging of prostate carcinoma. Clin Nucl Med. 2015;40:328–9. doi:10.1097/RLU.0000000000000709.
Avanesov M, Karul M, Derlin T. (68)Ga-PSMA as a new tracer for evaluation of prostate cancer: comparison between PET-CT and PET-MRI in biochemical recurrence. Radiologe. 2015;55:89–91. doi:10.1007/s00117-014-2792-6.
Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. doi:10.1007/s00259-014-2949-6.
Uprimny C, Kroiss A, Nilica B, et al. (68)Ga-PSMA ligand PET versus (18)F-NaF PET: evaluation of response to (223)Ra therapy in a prostate cancer patient. Eur J Nucl Med Mol Imaging. 2015;42:362–3. doi:10.1007/s00259-014-2922-4.
Iwata R, Pascali C, Bogni A, Furumoto S, Terasaki K, Yanai K. [18F]fluoromethyl triflate, a novel and reactive [18F]fluoromethylating agent: preparation and application to the on-column preparation of [18F]fluorocholine. Appl Radiat Isot. 2002;57:347–52.
Evangelista L, Cervino AR, Guttilla A, Zattoni F, Cuccurullo V, Mansi L. (1)(8)F-fluoromethylcholine or (1)(8)F-fluoroethylcholine pet for prostate cancer imaging: which is better? a literature revision. Nucl Med Biol. 2015;42:340–8. doi:10.1016/j.nucmedbio.2014.12.019.
Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pen tanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–53. doi:10.1158/1078-0432.CCR-11-1357.
Malik N, Baur B, Winter G, Reske SN, Beer AJ, Solbach C. Radiofluorination of PSMA-HBED via AlF chelation and biological evaluations in vitro. Mol Imaging Biol. 2015. doi:10.1007/s11307-015-0844-6.
Mease RC, Dusich CL, Foss CA, et al. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin Cancer Res. 2008;14:3036–43. doi:10.1158/1078-0432.CCR-07-1517.
McBride WJ, Sharkey RM, Goldenberg DM. Radiofluorination using aluminum-fluoride (Al18F). EJNMMI Res. 2013;3:36. doi:10.1186/2191-219X-3-36.
McBride WJ, D’Souza CA, Karacay H, Sharkey RM, Goldenberg DM. New lyophilized kit for rapid radiofluorination of peptides. Bioconjug Chem. 2012;23:538–47. doi:10.1021/bc200608e.
D’Souza CA, McBride WJ, Sharkey RM, Todaro LJ, Goldenberg DM. High-yielding aqueous 18F-labeling of peptides via Al18F chelation. Bioconjug Chem. 2011;22:1793–803. doi:10.1021/bc200175c.
Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–7.
Repetto-Llamazares AH, Larsen RH, Mollatt C, Lassmann M, Dahle J. Biodistribution and dosimetry of (177)Lu-tetulomab, a new radioimmunoconjugate for treatment of non-Hodgkin lymphoma. Curr Radiopharm. 2013;6:20–7.
Sparks RB, Aydogan B. Comparison of the effectiveness of some common animal data scaling techniques in estimating human radiation dose. . In: Stelson A, Stabin M, Sparks R, editors. Sixth International Radiopharmaceutical Dosimetry Symposium 1999. p. 705–16.
International Commission on Radiological Protection (ICRP). Publication 89: basic anatomical and physiological data for use in radiological protection—reference values. Ann ICRP. 2002;32:1–278.
Bitar A, Lisbona A, Thedrez P, et al. A voxel-based mouse for internal dose calculations using Monte Carlo simulations (MCNP). Phys Med Biol. 2007;52:1013–25. doi:10.1088/0031-9155/52/4/010.
Kletting P, Schimmel S, Kestler HA, et al. Molecular radiotherapy: the NUKFIT software for calculating the time-integrated activity coefficient. Med Phys. 2013;40:102504. doi:10.1118/1.4820367.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Dietlein M, Kobe C, Kuhnert G, et al. Comparison of [F]DCFPyL and [Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015. doi:10.1007/s11307-015-0866-0.
Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015. doi:10.1007/s11307-015-0850-8.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Fuchs AV, Tse BW, Pearce AK, et al. Evaluation of polymeric nanomedicines targeted to PSMA: effect of ligand on targeting efficiency. Biomacromolecules. 2015;16:3235–47. doi:10.1021/acs.biomac.5b00913.
Kochuparambil ST, Al-Husein B, Goc A, Soliman S, Somanath PR. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J Pharmacol Exp Ther. 2011;336:496–505. doi:10.1124/jpet.110.174870.
Horoszewicz JS, Leong SS, Kawinski E, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–18.
Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The novel theranostic PSMA-ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry and first evaluation of tumor lesions. J Nucl Med. 2015. doi:10.2967/jnumed.115.161299.
Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. doi:10.1007/s00259-013-2525-5.
Lavergne V, Ghannoum M, Christie M, et al. Risk factors and consequences of hyperaluminemia in a peritoneal dialysis cohort. Perit Dial Int. 2012;32:645–51. doi:10.3747/pdi.2011.00203.
Acknowledgment
We thank Larry Pang and Andreea Stuparu (members of the Ahmanson Translational Imaging Division) for their support and assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was partly supported by an unrestricted grant from the Movember Foundation—MOVEMBER GAP2 FUNDING AWARD.
Conflict of interest
Johannes Czernin is founder of Sofie Biosciences, which manufactures the Genisys4 scanner used in this manuscript. No other potential conflicts of interest are reported.
Ethical approval
Animal studies were approved by the UCLA Animal Research Committee and were carried out according to the guidelines of the Division of Laboratory Animal Medicine at UCLA. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants.
Author’s contributions
Initials: Stefano Boschi (SB), Jason T. Lee (JTL), Seval Beykan (SeB), Roger Slavik (RS), Liu Wei (LW), Uta Eberlein (UE), Andreas K. Buck (AKB), Filippo Lodi (FL), Gianfranco Coria (GC), Johannes Czernin (JC), Michael Lassmann (ML), Ken Herrmann (KH), Stefano Fanti (SF)
Conception and design: SB, AKB, JC, ML, KH, SF
Development of methodology: SB, JTL, RS, GC, ML, KH, SF
Acquisition of data: SB, JTL, RS, LW, FL, GC
Analysis and interpretation of data: JTL, SeB, RS, ML, KH, SF
Writing, review, and/or revision of the manuscript: all authors
Administrative, technical, or material support: SB, AKB, JC, ML, KH, SF
Supervision: SB, JC, ML, KH, SF
Additional information
Stefano Boschi and Jason T. Lee contributed equally to this work.
Rights and permissions
About this article
Cite this article
Boschi, S., Lee, J.T., Beykan, S. et al. Synthesis and preclinical evaluation of an Al18F radiofluorinated GLU-UREA-LYS(AHX)-HBED-CC PSMA ligand. Eur J Nucl Med Mol Imaging 43, 2122–2130 (2016). https://doi.org/10.1007/s00259-016-3437-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3437-y