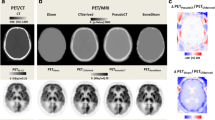
Abstract
Introduction
The combination of Positron Emission Tomography (PET) with magnetic resonance imaging (MRI) in hybrid PET/MRI scanners offers a number of advantages in investigating brain structure and function. A critical step of PET data reconstruction is attenuation correction (AC). Accounting for bone in attenuation maps (μ-map) was shown to be important in brain PET studies. While there are a number of MRI-based AC methods, no systematic comparison between them has been performed so far. The aim of this work was to study the different performance obtained by some of the recent methods presented in the literature. To perform such a comparison, we focused on [18F]-Fluorodeoxyglucose-PET/MRI neurodegenerative dementing disorders, which are known to exhibit reduced levels of glucose metabolism in certain brain regions.
Methods
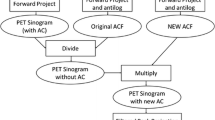
Four novel methods were used to calculate μ-maps from MRI data of 15 patients with Alzheimer’s dementia (AD). The methods cover two atlas-based methods, a segmentation method, and a hybrid template/segmentation method. Additionally, the Dixon-based and a UTE-based method, offered by a vendor, were included in the comparison. Performance was assessed at three levels: tissue identification accuracy in the μ-map, quantitative accuracy of reconstructed PET data in specific brain regions, and precision in diagnostic images at identifying hypometabolic areas.
Results
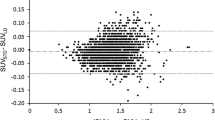
Quantitative regional errors of −20–−10 % were obtained using the vendor’s AC methods, whereas the novel methods produced errors in a margin of ±5 %. The obtained precision at identifying areas with abnormally low levels of glucose uptake, potentially regions affected by AD, were 62.9 and 79.5 % for the two vendor AC methods, the former ignoring bone and the latter including bone information. The precision increased to 87.5–93.3 % in average for the four new methods, exhibiting similar performances.
Conclusion
We confirm that the AC methods based on the Dixon and UTE sequences provided by the vendor are inferior to alternative techniques. As a novel finding, there was no substantial difference between the recently proposed atlas-based, template-based and segmentation-based methods.





Similar content being viewed by others
References
Kawachi T, Ishii K, Sakamoto S, et al. Comparison of the diagnostic performance of FDG-PET and VBM-MRI in very mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2006;33(7):801–9.
Teipel S, Drzezga A, Grothe M, et al. Multimodal imaging in Alzheimer’s disease: validity and usefulness for early detection. Lancet Neurol. 2015;14(10):1037–53.
Grimm R, Fürst S, Souvatzoglou M, et al. Self-gated MRI motion modelling for respiratory motion compensation in integrated PET/MRI. Med Image Anal. 2015;19(1):110–20.
Bai B, Li Q, Leahy RM. MR guided PET image reconstruction. Semin Nucl Med. 2013;43(1):30–44.
Hofmann M, Pichler B, Schölkopf B, Beyer T. Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques. Eur J Nucl Med Mol Imaging. 2009;36:S93–104.
Wagenknecht G, Kaiser HJ, Mottaghy FM, Herzog H. MRI for attenuation correction in PET: methods and challenges. MAGMA. 2013;26(1):99–113.
Keereman V, Mollet P, Berker Y, Schulz V, Vandenberghe S. Challenges and current methods for attenuation correction in PET/MR. MAGMA. 2013;26(1):81–98.
Visvikis D, Monnier F, Bert J, Hatt M, Fayad H. PET/MR attenuation correction: where have we come from and where are we going? Eur J Nucl Med Mol Imaging. 2014;41(6):1172–5.
Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189–94.
Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr. 2003;27(6):825–46.
Sekine T, Buck A, Delso G, et al. Evaluation of atlas-based attenuation correction for integrated PET/MR in human brain - application of a head atlas and comparison to true CT-based attenuation correction. J Nucl Med. 2016;57(2):215–20.
Dickson JC, O’Meara C, Barnes A. A comparison of CT- and MR-based attenuation correction in neurological PET. Eur J Nucl Med Mol Imaging. 2014;41:1176–89.
Andersen FL, Ladefoged CN, Beyer T, et al. Combined PET/MR imaging in neurology: MR-based attenuation correction implies a strong spatial bias when ignoring bone. NeuroImage. 2014;84:206–16.
Nuyts J, Dupont P, Stroobants S, Benninck R, Mortelmans L, Suetens P. Simultaneous maximum a posteriori reconstruction of attenuation and activity distributions from emission sonograms. IEEE Trans Med Imaging. 1999;18:393–403.
Rezaei A, Defrise M, Bal G, Michel C, Conti M, Watson C, et al. Simultaneous reconstruction of activity and attenuation in time-of-flight PET. IEEE Trans Med Imaging. 2012;31:2224–33.
Aitken AP, Giese D, Tsoumpas C, et al. Improved UTE-based attenuation correction for cranial PET-MR using dynamic magnetic field monitoring. Med Phys. 2014;41(1):012302.
Delso G, Wiesinger F, Sacolick LI, et al. Clinical evaluation of zero-echo-time MR imaging for the segmentation of the skull. J Nucl Med. 2015;56(3):417–22.
Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med. 2012;67(2):510–8.
Santos Ribeiro A, Rota-Kops E, Herzog H, Almeida P. Hybrid approach for attenuation correction in PET/MR scanners. Nucl Inst Methods Phys Res A. 2014;A734:166–70.
Delso G, Zeimpekis K, Carl F, Wiesinger F, Hüllner M, Veit-Haibach P. Cluster-based segmentation of dual-echo ultra-short echo time images for PET/MR bone localization. Eur J Nucl Med Mol Imaging. 2014;1:7.
Roy S, Wang WT, Carass A, Prince JL, Butman JA, Pham DL. PET attenuation correction using synthetic CT from ultrashort echo-time MR imaging. J Nucl Med. 2014;55(12):2071–7.
Burgos N, Cardoso MJ, Thieleman K, et al. Attenuation correction synthesis for hybrid PET-MR scanners: application to brain studies. IEEE Trans Med Imaging. 2014;33:2332–41.
Hofmann M, Steinke F, Scheel V, et al. MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med. 2008;49:1875–83.
Navalpakkam BK, Braun H, Kuwert T, Quick HH. Magnetic resonance-based attenuation correction for PET/MR hybrid imaging using continuous valued attenuation maps. Investig Radiol. 2013;48(5):323–32.
Johansson A, Karlsson M, Nyholm T. CT substitute derived from MRI sequences with ultrashort echo time. Med Phys. 2011;38(5):2708–14.
Izquierdo-Garcia D, Hansen AE, Förster S, et al. An SPM8-based approach for attenuation correction combining segmentation and non-rigid template formation: application to simultaneous PET/MR brain imaging. J Nucl Med. 2014;55:1825–30.
Keereman V, Fierens Y, Broux T, De Deene Y, Lonneux M, Vandenberghe S. MRI-based attenuation correction for PET/MRI using ultrashort echo time sequences. J Nucl Med. 2010;51:812–8.
Cabello J, Lukas M, Förster S, et al. MR-based attenuation correction using ultrashort-echo-time pulse sequences in dementia patients. J Nucl Med. 2015;56(3):423–9.
Choi H, Cheon GJ, Kin HJ, et al. Segmentation-based MR attenuation correction including bones also affects quantitation in brain studies: an initial result of 18F-FP-CIT PET/MR for patients with parkinsonism. J Nucl Med. 2014;55(10):1617–22.
Catana C, van der Kouwe A, Benner T, et al. Toward implementing an MRI based PET attenuation-correction method for neurologic studies on the MR-PET brain prototype. J Nucl Med. 2010;51:1431–8.
Berker Y, Franke J, Salomon A, et al. MRI-based attenuation correction for hybrid PET/MRI systems: a 4-class tissue segmentation technique using a combined ultrashort-echo-time/Dixon MRI sequence. J Nucl Med. 2012;53:796–804.
Schulz V, Torres-Espallardo I, Renisch S, et al. Automatic, three-segment, MR-based attenuation correction for whole-body PET/MR data. Eur J Nucl Med Mol Imaging. 2011;38(1):138–52.
Delso G, Carl M, Wiesinger F, et al. Anatomic evaluation of 3-dimensional ultrashort-echo-time bone maps for PET/MR attenuation correction. J Nucl Med. 2014;55:780–5.
Schaefer SM, Abercrombie HC, Lindgren KA, et al. Six-month test–retest reliability of MRI-defined PET measures of regional cerebral glucose metabolic rate in selected subcortical structures. Hum Brain Mapp. 2000;19(1):1–9.
Camargo EE, Szabo Z, Links JM, Sostre S, Dannals RF, Wagner JRHN. The influence of biological and technical factors on the variability of global and regional brain metabolism of 2-[18F]Fluoro-2-deoxy-D-glucose. J Cereb Blood Flow Metab. 1992;12:281–90.
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–48.
Delso G, Fürst S, Jakoby B, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52:1914–22.
Watson CC, Rappoport V, Faul D, Townsend DW, Carney JP. A method for calibrating the CT-based attenuation correction of PET in human tissue. IEEE Trans Nucl Sci. 2006;53:102–7.
Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;3:165–89.
Ladefoged CN, Hansen AE, Kelelr SH, et al. Dental artifacts in the head and neck region: implications for Dixon-based attenuation correction in PET/MR. Eur J Nucl Med Mol Imaging. 2015;2:8.
Acknowledgments
The PET/MRI facility at the Technische Universität München was funded by the Großgeräteinitiative from the Deutsche Forschungsgemeinschaft, (DFG). The research leading to these results has received funding from the European Union Seventh Framework Program (FP7) under Grant Agreement n° 602621- Trimage, n° 294582- MUMI, and from the DFG grant no. FO 886/1–1. We thank David Izquierdo and Ninon Burgos for their assistance using their algorithms. We also thank Sylvia Schachoff and Claudia Meisinger for their technical assistance with the PET/MRI scanner.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The research leading to these results has received funding from the Seventh Framework Programme [FP7/2007-2013] under grant agreement n° 602621- Trimage, n° 294582- MUMI, and from the DFG grant no. FO 886/1–1.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(DOCX 181 kb)
Supplementary Fig. 2
(DOCX 434 kb)
Supplementary Fig. 3
(DOCX 100 kb)
Supplementary Fig. 4
(DOCX 110 kb)
Supplementary Fig. 5
(DOCX 345 kb)
Supplementary Fig. 6
(DOCX 238 kb)
Supplementary Table 1
(DOCX 16 kb)
Supplementary Table 2
(DOCX 16 kb)
Supplementary Table 3
(DOCX 18 kb)
Supplementary Table 4
(DOCX 16 kb)
Supplementary Table 5
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Cabello, J., Lukas, M., Rota Kops, E. et al. Comparison between MRI-based attenuation correction methods for brain PET in dementia patients. Eur J Nucl Med Mol Imaging 43, 2190–2200 (2016). https://doi.org/10.1007/s00259-016-3394-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3394-5