Abstract
Purpose
We evaluated whether 18F-3′-deoxy-3′-fluorothymidine positron emission tomography (FLT PET) can predict the final postoperative histopathological response in primary breast cancer after the first cycle of neoadjuvant chemotherapy (NCT).
Methods
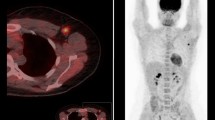
In this prospective cohort study of 15 patients with locally advanced operable breast cancer, FLT PET evaluations were performed before NCT, after the first cycle of NCT, and at the end of NCT. All patients subsequently underwent surgery. Variables from FLT PET examinations were correlated with postoperative histopathological results.
Results
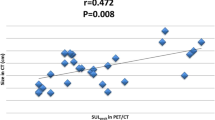
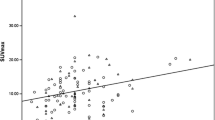
At baseline, median of maximum standardized uptake values (SUVmax) in the groups showing a complete pathological response (pCR) + residual cancer burden (RCB) I, RCB II or RCB III did not differ significantly for the primary tumour (5.0 vs. 2.9 vs. 8.9, p = 0.293) or for axillary nodes (7.9 vs. 1.6 vs. 7.0, p = 0.363), whereas the Spearman correlation between SUVmax and Ki67 proliferation rate index was significant (r = 0.69, p < 0.001). Analysis of the relative percentage change of SUVmaxin the primary tumour (∆SUVTmax(t 1)) and axillary nodes (∆SUVNmax(t 1)) after the first NCT cycle showed that the power of ∆SUVTmax(t 1) to predict pCR + RCB I responses (AUC = 0.91, p < 0.001) was statistically significant, whereas ∆SUVNmax(t 1) had a moderate ability (AUC = 0.77, p = 0.119) to separate subjects with ΔSUVTmax(t 1) > −52.9 % into two groups: RCB III patients and a heterogeneous group that included RCB I and RCB II patients. A predictive score μ based on ΔSUVTmax(t 1) and ΔSUVNmax(t 1) parameters is proposed.
Conclusion
The preliminary findings of the present study suggest the potential utility of FLT PET scans for early monitoring of response to NCT and to formulate a therapeutic strategy consistent with the estimated efficacy of NCT. However, these results in a small patient population need to be validated in a larger independent cohort.





Similar content being viewed by others
References
Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93.
Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93–100.
von Minckwitz G, Raab G, Caputo A, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005;23:2676–85.
Gianni G, Baselga J, Eiermann W, et al. Feasibility and tolerability of sequential doxorubicin/paclitaxel followed by cyclophosphamide, methotrexate, and fluorouracil and its effects on tumor response as preoperative therapy. Clin Cancer Res. 2005;11:8715–21.
von Minckwitz G, Untch M, Blohmer J, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL-CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–9.
Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg. 1995;180:297–306.
Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22.
Kaufmann M, Hortobagyi GN, Goldhirsch A, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24:1940–9.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85.
Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an International Consensus Conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–16.
Corben AD, Abi-Raad R, Popa I, et al. Pathologic response and long-term follow-up in breast cancer patients treated with neoadjuvant chemotherapy. A comparison between classifications and their practical application. Arch Pathol Lab Med. 2013;137:1074–82.
Schott AF, Roubidoux MA, Helvie MA, et al. Clinical and radiologic assessments to predict breast cancer pathologic complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2005;92:231–8.
O’Flynn EA, Desousa NM. Functional magnetic resonance: biomarkers of response in breast cancer. Breast Cancer Res. 2011;13:204–40.
Le-Petross HC, Hylton N. Role of breast MR imaging in neoadjuvant chemotherapy. Magn Reson Imaging Clin N Am. 2010;18:249–58.
Li X, Abramson RG, Arlinghaus LR, et al. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol. 2014. doi:10.1097/RLI.0000000000000100
Jacobs MA, Ouwerkerk R, Wolff AC, et al. Monitoring of neoadjuvant chemotherapy using multiparametric, 23Na sodium MR, and multimodality (PET/CT/MRI) imaging in locally advanced breast cancer. Breast Cancer Res Treat. 2011;128:119–26.
Manton DJ, Chaturvedi A, Hubbard A, et al. Neoadjuvant chemotherapy in breast cancer: early response prediction with quantitative MR imaging and spectroscopy. Br J Cancer. 2006;94:427–35.
Meisamy S, Bolan PJ, Baker EH, et al. Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo 1H MR spectroscopy – a pilot study at 4 T. Radiology. 2004;233:424–31.
Cochet A, Generali D, Fox SB, Ferrozzi F, Hicks RJ. Positron emission tomography and neoadjuvant therapy of breast cancer. J Natl Cancer Inst Monogr. 2011;43:111–5.
Barriolo-Riedinger A, Touzery C, Riedinger JM, et al. [18F]FDG-PET predicts complete pathological response of breast cancer to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2007;34:1915–24.
Duch J, Fuster D, Munoz M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36:1551–7.
Groheux D, Giacchetti S, Hatt M, et al. HER2-overexpressing breast cancer: FDG uptake after two cycles of chemotherapy predicts the outcome of neoadjuvant treatment. Br J Cancer. 2013;109:1157–64.
Groheux D, Hindie E, Giacchetti S, et al. Early assessment with 18F-fluorodeoxyglucose positron emission tomography/computed tomography can help predict the outcome of neoadjuvant chemotherapy in triple negative breast cancer. Eur J Cancer. 2014;50:1864–71.
Koolen BB, Pengel KE, Wesseling J, et al. FDG PET/CT during neoadjuvant chemotherapy may predict response in ER-positive/HER2-negative and triple negative, but not in HER2-positive breast cancer. Breast. 2013;22:691–7.
Humbert O, Berroilo-Riedinger A, Riedinger JM, et al. Changes in 18F-FDG tumor metabolism after a first course of neoadjuvant chemotherapy in breast cancer: influence of tumor subtypes. Ann Oncol. 2012;23:2572–7.
Koolen BB, Pengel KE, Wesseling J, et al. Sequential 18F-FDG PET/CT for early prediction of complete pathological response in breast and axilla during neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:32–40.
Gebhart G, Gamez C, Holmes E, et al. 18F-FDG PET/CT for early prediction of response of neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J Nucl Med. 2013;54:1862–8.
Andrade WP, Lima ENP, Osorio CABT, et al. Can FDG-PET/CT predict early response to neoadjuvant chemotherapy in breast cancer? Eur J Surg Oncol. 2013;39:1358–63.
Salskov A, Tammisetti VS, Grierson J, Vesselle H. FLT: measuring tumor cell proliferation in vivo with positron emission tomography and 3′-deoxy-3′-18F-fluorothymidine. Semin Nucl Med. 2007;37:429–39.
Rasey JS, Grierson JR, Wiens LW, et al. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43:1210–7.
Been LB, Suurmeijer AJ, Cobben DCP, Elsinga PH, de Vries J, et al. [18F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Mol Imaging. 2004;31:1659–72.
Pio BS, Park CK, Pietras R, et al. Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol. 2006;8:36–42.
Kenny L, Coombes RC, Vigushin DM, Al-Nahhas A, Shousha S, Aboagye EO. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-18F-fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging. 2007;34:1339–47.
Contractor KB, Kenny LM, Stebbing J, et al. [18F]-3′deoxy-3′-fluorothymidine positron emission tomography and breast cancer response to docetaxel. Clin Cancer Res. 2011;17:7664–72.
Woolf DK, Beresford M, Li SP, et al. Evaluation of FLT-PET-CT as imaging biomarker of proliferation in primary breast cancer. Br J Cancer. 2014;110:2847–54.
Pascali C, Bogni A, Fugazza L, et al. Simple preparation and purification of ethanol-free solutions of 3'-deoxy-3'-[18F]fluorothymidine by means of disposable solid-phase extraction cartridges. Nucl Med Biol. 2012;39:540–50.
Smyczek-Gargya B, Fersis N, Dittmann H, et al. PET with [18F]fluorothymidine for imaging of primary breast cancer: a pilot study. Eur J Nucl Med. 2004;31:720–4.
The University of Texas MD Anderson Cancer Center. Residual cancer burden calculator. http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3. Accessed 29 Jan 2015.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23.
Farwell MD, Pryma DA, Mankoff DA. PET/CT imaging in cancer: current applications and future directions. Cancer. 2014;120:3433–45.
Schwartz JL, Tamura Y, Jordan R, Grierson JR, Krohn KA. Monitoring tumor cell proliferation by targeting DNA synthetic processes with thymidine and thymidine analogs. J Nucl Med. 2003;44:2027–32.
Kenny LM, Vigushin DM, Al-Nahhas A, et al. Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by 18F-fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res. 2005;65:10104–12.
Buck AK, Bommer M, Stilgenbauer S, et al. Molecular imaging of proliferation in malignant lymphoma. Cancer Res. 2006;66:11055–61.
Yamamoto Y, Nishiyama Y, Ishikawa S, et al. Correlation of 18F-FLT and 18F-FDG uptake on PET with Ki-67 immunochemistry in non-small cell lung cancer. Eur J Med Mol Imaging. 2007;34:1610–6.
Chalkidou A, Landau DB, Odell EW, et al. Correlation between Ki-67 immunohistochemistry and 18F-Fluorothymidine uptake in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2012;48:3499–513.
Dittmann H, Dohmen BM, Paulsen F, et al. [18F]FLT PET for diagnosis and staging of thoracic tumours. Eur J Nucl Med Mol Imaging. 2003;30:1407–12.
Buck AK, Halter G, Schirrmeister H, et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med. 2003;44:1426–31.
Hoshikawa H, Nishiyama Y, Kishino T, et al. Comparison of FLT-PET and FDG-PET for visualization of head and neck squamous cell cancers. Mol Imaging Biol. 2011;13:172–7.
Kameyama R, Yamamoto Y, Izuishi K, et al. Detection of gastric cancer using 18F-FLT PET: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2009;36:382–8.
Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46:945–52.
van Westreenen HL, Cobben DCP, Jager PL, et al. Comparison of 18F-FLT PET and 18F-FDG in esophageal cancer. J Nucl Med. 2005;46:400–4.
van Waarde A, Cobben DC, Suurmeijer AJ, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumour from inflammation in a rodent model. J Nucl Med. 2004;45:695–700.
Kenny LM, Al-Nahhas A, Aboagye EO. Novel PET biomarkers for breast cancer imaging. Nucl Med Commun. 2011;32:333–5.
Lubberink M, Direcks W, Emmering J, et al. Validity of simplified 3′-deoxy-3′-[18F]fluorothymidine uptake measures for monitoring response to chemotherapy in locally advanced breast cancer. Mol Imaging Biol. 2012;14:777–82.
Kinahan PE, Fletcher JW. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MRI. 2010;31:496–505.
Eckel F, Herrmann K, Schmidt S, et al. Imaging of proliferation in hepatocellular carcinoma with the in vivo marker 18F-fluorothymidine. J Nucl Med. 2009;50:1441–7.
Buck AK, Schirrmeister H, Hetzel M, et al. 3-deoxy-3-[(18)F]fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res. 2002;62:3331–4.
Vesselle H, Grierson J, Muzi M, et al. In vivo validation of 3-deoxy-3-18F-fluorothymidine (18F-FLT) as a proliferation imaging tracer in humans: correlation of 18F-FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–23.
Wang Y, Zhang C, Liu J, Huang G. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res Treat. 2012;131:357–69.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72.
Provenzano E, Vallier A-L, Champ R, et al. A central review of histopathology reports after breast cancer neoadjuvant chemotherapy in the neo-tango trial. Br J Cancer. 2013;108:866–72.
Ogston KN, Miller ID, Payne S, et al. A new histologic grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12:320–7.
Chollet P, Abrial C, Durando X, et al. A new prognostic classification after primary chemotherapy for breast cancer: residual disease in breast and nodes (RDBN). Cancer J. 2008;14:128–32.
Marchiò C, Sapino A. The pathologic complete response open question in primary therapy. J Natl Cancer Inst Monogr. 2011;43:86–90.
Kim S, Kim DH, Jung WH, et al. Metabolic phenotypes in triple negative breast cancer. Tumor Biol. 2013;34:1699–712.
Kim SK, Jung WH, Koo JS. Differential expression of enzymes associated with serine/glycine metabolism in different breast cancer subtypes. PLoS One. 2014;9:e101004.
Kajari K, Tokes T, Dank M, et al. Correlation of the value of 18F-FDG uptake, described by SUVmax, SUVavg, metabolic tumour volume and total lesion glycolysis, to clinicopathological prognostic factors and biological subtypes in breast cancer. Nucl Med Commun. 2015;36:28–37.
Vicente G, Castrejon AS, Leon MA, et al. Molecular subtypes of breast cancer: metabolic correlation with 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:1304–11.
Wu L, Hu J, Gu H, et al. Can diffusion weighted MR imaging and contrast-enhanced MR imaging precisely evaluate and predict pathological response to neoadjuvant chemotherapy in patients with breast cancer? Breast Cancer Res Treat. 2012;135:17–28.
Pengel KE, Koolen BB, Loo CE, et al. Combined use of 18F-FDG PET/CT and MRI for response monitoring of breast cancer during neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1515–24.
Partridge SC, Vanantwerp RK, Doot RK, et al. Association between serial dynamic contrast enhanced MRI and dynamic 18F-FDG PET measures in patients undergoing neoadjuvant chemotherapy for locally advanced breast cancer. J Magn Reson Imaging. 2010;32:1124–31.
Bahri S, Chen J, Mehta RS, et al. Residual breast cancer diagnosed by MRI in patients receiving neoadjuvant chemotherapy with and without bevacizumab. Cancer. 2009;16:1619–28.
Conflicts of interest
None.
Funding
This work was supported by a grant from Associazione Italiana Ricerca sul Cancro (Study INT/35/10).
Author information
Authors and Affiliations
Corresponding author
Additional information
Flavio Crippa and Roberto Agresti contributed equally to this work.
Rights and permissions
About this article
Cite this article
Crippa, F., Agresti, R., Sandri, M. et al. 18F-FLT PET/CT as an imaging tool for early prediction of pathological response in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy: a pilot study. Eur J Nucl Med Mol Imaging 42, 818–830 (2015). https://doi.org/10.1007/s00259-015-2995-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-2995-8