Abstract
Purpose
In a previous study, we demonstrated the first evidence that the asphericity (ASP) of pretherapeutic FDG uptake in the primary tumor provides independent prognostic information in patients with head and neck cancer. The aim of this work was to confirm these results in an independent patient group examined at a different site.
Methods
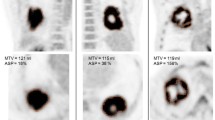
FDG-PET/CT was performed in 37 patients. The primary tumor was delineated by an automatic algorithm based on adaptive thresholding. For the resulting ROIs, the metabolically active part of the tumor (MTV), SUVmax, SUVmean, total lesion glycolysis (TLG) and ASP were computed. Univariate Cox regression with respect to progression free survival (PFS) and overall survival (OS) was performed. For survival analysis, patients were divided in groups of high and low risk according to the parameter cut-offs defined in our previous work. In a second step, the cut-offs were adjusted to the present data. Univariate and multivariate Cox regression was performed for the pooled data consisting of the current and the previously described patient group (N = 68). In multivariate Cox regression, clinically relevant parameters were included.
Results
Univariate Cox regression using the previously published cut-off values revealed TLG (hazard ratio (HR) = 3) and ASP (HR = 3) as significant predictors for PFS. For OS MTV (HR = 2.7) and ASP (HR = 5.9) were significant predictors. Using the adjusted cutoffs MTV (HR = 2.9/3.3), TLG (HR = 3.1/3.3) and ASP (HR = 3.1/5.9) were prognostic for PFS/OS. In the pooled data, multivariate Cox regression revealed a significant prognostic value with respect to PFS/OS for MTV (HR = 2.3/2.1), SUVmax (HR = 2.1/2.5), TLG (HR = 3.5/3.6), and ASP (HR = 3.4/4.4).
Conclusions
Our results confirm the independent prognostic value of ASP of the pretherapeutic FDG uptake in the primary tumor in patients with head and neck cancer. Moreover, these results demonstrate that ASP can be determined unambiguously across different sites.



Similar content being viewed by others
References
Wahl R, Jacene H, Kasamon Y, Lodge M. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S.
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging: the visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2(3):159–71.
Guillem JG, Moore HG, Akhurst T, Klimstra DS, Ruo L, Mazumdar M, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining long-term outcomes of rectal cancer. J Am Coll Surg. 2004;199(1):1–7.
Francis RJ, Byrne MJ, van der Schaaf AA, Boucek JA, Nowak AK, Phillips M, et al. Early prediction of response to chemotherapy and survival in malignant pleural mesothelioma using a novel semiautomated 3-dimensional volume-based analysis of serial 18F-FDG PET scans. J Nucl Med. 2007;48(9):1449–58.
Apostolova I, Steffen IG, Wedel F, Lougovski A, Marnitz S, Derlin T, et al. Asphericity of pretherapeutic tumour FDG uptake provides independent prognostic value in head-and-neck cancer. Eur Radiol. 2014;24(9):2077–87.
Wadell H. Volume, shape, and roundness of quartz particles. J Geol. 1935:250–80.
Hentschel M, Appold S, Schreiber A, Abolmaali N, Abramyuk A, Dörr W, et al. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2011;38(7):1203–11.
Hofheinz F, Pötzsch C, Oehme L, Beuthien-Baumann B, Steinbach J, Kotzerke J, et al. Automatic volume delineation in oncological PET. Evaluation of a dedicated software tool and comparison with manual delineation in clinical data sets. Nuklearmedizin. 2012;51(1):9–16.
R-Development-Core-Team. R. 2008. A language and environment for statistical computing. R Foundation for Statistical Computing. ISBN 3-900051-07-0.
Chan SC, Chang JT, Lin CY, Ng SH, Wang HM, Liao CT, et al. Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for different survival endpoints and impact on prognostic stratification. Nucl Med Commun. 2011;32(11):989–96.
Dibble EH, Alvarez ACL, Truong MT, Mercier G, Cook EF, Subramaniam RM. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. 2012;53(5):709–15.
Lim R, Eaton A, Lee NY, Setton J, Ohri N, Rao S, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med. 2012;53(10):1506–13.
Ryu IS, Kim JS, Roh JL, Lee JH, Cho KJ, Choi SH, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis measured by 18F-FDG PET/CT in salivary gland carcinomas. J Nucl Med. 2013;54(7):1032–38.
Erdi Y, Mawlawi O, Larson S, Imbriaco M, Yeung H, Finn R, et al. Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer 1997;80(s 12):2505–9.
Daisne J, Sibomana M, Bol A, Doumont T, Lonneux M, Gregoire V. Tri-dimensional automatic segmentation of PET volumes based on measured source-to-background ratios: influence of reconstruction algorithms. Radiother Oncol. 2003;69(3):247–50.
Boellaard R, Krak N, Hoekstra O, Lammertsma A. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45(9):1519–27.
Black Q, Grills I, Kestin L, Wong C, Wong J, Martinez A, et al. Defining a radiotherapy target with positron emission tomography. Int J Radiat Oncol Biol Phys. 2004;60(4):1272–82.
Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rübe C, et al. Comparison of different methods for delineation of 18 F-FDG PET–positive tissue for target volume definition in radiotherapy of patients with non–small cell lung cancer. J Nucl Med. 2005;46(8):1342–8.
Drever L, Robinson D, McEwan A, Roa W. A local contrast based approach to threshold segmentation for PET target volume delineation. Med Phys. 2006;33:1583.
van Dalen J, Hoffmann A, Dicken V, Vogel W, Wiering B, Ruers T, et al. A novel iterative method for lesion delineation and volumetric quantification with FDG PET. Nucl Med Commun 2007;28(6):485–93.
Jentzen W, Freudenberg L, Eising E, Heinze M, Brandau W, Bockisch A. Segmentation of PET volumes by iterative image thresholding. J Nucl Med. 2007;48(1):108–14.
Vauclin S, Doyeux K, Hapdey S, Edet-Sanson A, Vera P, Gardin I. Development of a generic thresholding algorithm for the delineation of 18FDG-PET-positive tissue: application to the comparison of three thresholding models. Phys Med Biol. 2009;54(22):6901–16.
Frings V, de Langen A, Smit E, van Velden F, Hoekstra O, van Tinteren H, et al. Repeatability of metabolically active volume measurements with 18F-FDG and 18F-FLT PET in non-small cell lung cancer. J Nucl Med. 2010;51(12):1870.
Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37(1):181–200.
Acknowledgments
This work was supported in parts by the German Federal Ministry of Education and Research (BMBF contract 03ZIK42/OncoRay).
Conflict of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Hofheinz and A. Lougovski contributed equally to this article.
Rights and permissions
About this article
Cite this article
Hofheinz, F., Lougovski, A., Zöphel, K. et al. Increased evidence for the prognostic value of primary tumor asphericity in pretherapeutic FDG PET for risk stratification in patients with head and neck cancer. Eur J Nucl Med Mol Imaging 42, 429–437 (2015). https://doi.org/10.1007/s00259-014-2953-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2953-x