Abstract
Purpose
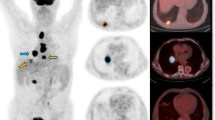
The aim of this study was to investigate correlations between glucose metabolism as determined by [18F]FDG PET/CT and tumour perfusion as quantified by volume perfusion CT in primary tumours and mediastinal lymph nodes (MLN) of patients with non-small-cell lung cancer (NSCLC).
Methods
Enrolled in the study were 17 patients with NSCLC. [18F]FDG uptake was quantified in terms of SUVmax and SUVavg. Blood flow (BF), blood volume (BV) and flow extraction product (Ktrans) were determined as perfusion parameters. The correlations between the perfusion parameters and [18F]FDG uptake values were subsequently evaluated.
Results
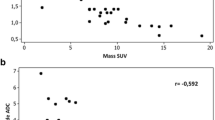
For the primary tumours, no correlations were found between perfusion parameters and [18F]FDG uptake. In MLN, there were negative correlations between BF and SUVavg (r = −0.383), BV and SUVavg (r = −0.406), and BV and SUVmax (r = −0.377), but not between BF and SUVmax, Ktrans and SUVavg, or Ktrans and SUVmax. Additionally, in MLN with SUVmax >2.5 there were negative correlations between BF and SUVavg (r = −0.510), BV and SUVavg (r = −0.390), BF and SUVmax (r = −0.536), as well as BV and SUVmax (r = −0.346).
Conclusion
Perfusion and glucose metabolism seemed to be uncoupled in large primary tumours, but an inverse correlation was observed in MLN. This information may help improve therapy planning and response evaluation.

Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Raz DJ, Zell JA, Ou SH, Gandara DR, Anton-Culver H, Jablons DM. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest. 2007;132:193–9.
Farray D, Mirkovic N, Albain KS. Multimodality therapy for stage III non-small-cell lung cancer. J Clin Oncol. 2005;23:3257–69.
Walker CM, Chung JH, Abbott GF, Little BP, El-Sherief AH, Shepard JA, et al. Mediastinal lymph node staging: from noninvasive to surgical. AJR Am J Roentgenol. 2012;199:W54–64.
Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132:178S–201.
Vansteenkiste JF, Stroobants SG, Dupont PJ, De Leyn PR, De Wever WF, Verbeken EK, et al. FDG-PET scan in potentially operable non-small cell lung cancer: do anatometabolic PET-CT fusion images improve the localisation of regional lymph node metastases? The Leuven Lung Cancer Group. Eur J Nucl Med. 1998;25:1495–501.
Schmid-Bindert G, Henzler T, Chu TQ, Meyer M, Nance Jr JW, Schoepf UJ, et al. Functional imaging of lung cancer using dual energy CT: how does iodine related attenuation correlate with standardized uptake value of 18FDG-PET-CT? Eur Radiol. 2012;22:93–103.
Mandeville HC, Ng QS, Daley FM, Barber PR, Pierce G, Finch J, et al. Operable non-small cell lung cancer: correlation of volumetric helical dynamic contrast-enhanced CT parameters with immunohistochemical markers of tumor hypoxia. Radiology. 2012;264:581–9.
de Langen AJ, van den Boogaart V, Lubberink M, Backes WH, Marcus JT, van Tinteren H, et al. Monitoring response to antiangiogenic therapy in non-small cell lung cancer using imaging markers derived from PET and dynamic contrast-enhanced MRI. J Nucl Med. 2011;52:48–55.
Schillaci O, Spanu A, Scopinaro F, Monteleone F, Solinas ME, Volpino P, et al. Mediastinal lymph node involvement in non-small cell lung cancer: evaluation with 99mTc-tetrofosmin SPECT and comparison with CT. J Nucl Med. 2003;44:1219–24.
Hoekstra CJ, Stroobants SG, Hoekstra OS, Smit EF, Vansteenkiste JF, Lammertsma AA. Measurement of perfusion in stage IIIA-N2 non-small cell lung cancer using H(2)(15)O and positron emission tomography. Clin Cancer Res. 2002;8:2109–15.
Graves EE, Maity A, Le QT. The tumor microenvironment in non-small-cell lung cancer. Semin Radiat Oncol. 2010;20:156–63.
Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006;12:1507–14.
Bollineni VR, Wiegman EM, Pruim J, Groen HJ, Langendijk JA. Hypoxia imaging using positron emission tomography in non-small cell lung cancer: implications for radiotherapy. Cancer Treat Rev. 2012;38:1027–32.
Veronesi G, Landoni C, Pelosi G, Picchio M, Sonzogni A, Leon ME, et al. Fluoro-deoxi-glucose uptake and angiogenesis are independent biological features in lung metastases. Br J Cancer. 2002;86:1391–5.
Hirasawa S, Tsushima Y, Takei H, Hirasawa H, Taketomi-Takahashi A, Takano A, et al. Inverse correlation between tumor perfusion and glucose uptake in human head and neck tumors. Acad Radiol. 2007;14:312–8.
Ohno Y, Koyama H, Matsumoto K, Onishi Y, Takenaka D, Fujisawa Y, et al. Differentiation of malignant and benign pulmonary nodules with quantitative first-pass 320-detector row perfusion CT versus FDG PET/CT. Radiology. 2011;258:599–609.
Rajendran JG, Mankoff DA, O’Sullivan F, Peterson LM, Schwartz DL, Conrad EU, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245–52.
Huang T, Civelek AC, Li J, Jiang H, Ng CK, Postel GC, et al. Tumor microenvironment-dependent 18F-FDG, 18F-fluorothymidine, and 18F-misonidazole uptake: a pilot study in mouse models of human non-small cell lung cancer. J Nucl Med. 2012;53:1262–8.
Hellwig D, Graeter TP, Ukena D, Groeschel A, Sybrecht GW, Schaefers HJ, et al. 18F-FDG PET for mediastinal staging of lung cancer: which SUV threshold makes sense? J Nucl Med. 2007;48:1761–6.
Miles KA, Hayball M, Dixon AK. Colour perfusion imaging: a new application of computed tomography. Lancet. 1991;337:643–5.
Tacelli N, Remy-Jardin M, Copin MC, Scherpereel A, Mensier E, Jaillard S, et al. Assessment of non-small cell lung cancer perfusion: pathologic-CT correlation in 15 patients. Radiology. 2010;257:863–71.
van den Hoff J. Assessment of lung cancer perfusion by using patlak analysis: what do we measure? Radiology. 2007;243:907–8.
Thieme SF, Johnson TR, Lee C, McWilliams J, Becker CR, Reiser MF, et al. Dual-energy CT for the assessment of contrast material distribution in the pulmonary parenchyma. AJR Am J Roentgenol. 2009;193:144–9.
Kahraman D, Scheffler M, Zander T, Nogova L, Lammertsma AA, Boellaard R, et al. Quantitative analysis of response to treatment with Erlotinib in advanced non-small cell lung cancer using 18F-FDG and 3′-deoxy-3′-18F-fluorothymidine PET. J Nucl Med. 2011;52:1871–7.
Yabuuchi H, Hatakenaka M, Takayama K, Matsuo Y, Sunami S, Kamitani T, et al. Non-small cell lung cancer: detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology. 2011;261:598–604.
Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76.
Acknowledgments
We wish to thank our technicians Nicole Sachse, Astrid Schreiber, Henriette Heners and Simeon Winterstein for excellent assistance. Siemens Healthcare supported the study.
Conflicts of interest
Claus D. Claussen has a collaboration contract with Siemens Healthcare concerning the development of volume perfusion CT. Ernst Klotz is a Siemens Healthcare employee. All other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sauter, A.W., Spira, D., Schulze, M. et al. Correlation between [18F]FDG PET/CT and volume perfusion CT in primary tumours and mediastinal lymph nodes of non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 40, 677–684 (2013). https://doi.org/10.1007/s00259-012-2318-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2318-2