Abstract
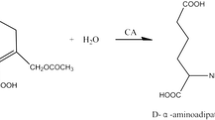
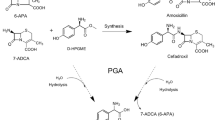
In this report, redesigning cephalosporin C acylase from the Pseudomonas strain N176 revealed that the loss of stability owing to the introduced mutations at the active site can be recovered by repacking the nearby hydrophobic core regions. Starting from a quadruple mutant M31βF/H57βS/V68βA/H70βS, whose decrease in stability is largely owing to the mutation V68βA at the active site, we employed a computational enzyme design strategy that integrated design both at hydrophobic core regions for stability enhancement and at the active site for activity improvement. Single-point mutations L154βF, Y167βF, L180βF and their combinations L154βF/L180βF and L154βF/Y167βF/L180βF were found to display improved stability and activity. The two-point mutant L154βF/L180βF increased the protein melting temperature (T m) by 11.7 °C and the catalytic efficiency V max/K m by 57 % compared with the values of the starting quadruple mutant. The catalytic efficiency of the resulting sixfold mutant M31βF/H57βS/V68βA/H70βS/L154βF/L180βF is recovered to become comparable to that of the triple mutant M31βF/H57βS/H70βS, but with a higher T m. Further experiments showed that single-point mutations L154βF, L180βF, and their combination contribute no stability enhancement to the triple mutant M31βF/H57βS/H70βS. These results verify that the lost stability because of mutation V68βA at the active site was recovered by introducing mutations L154βF and L180βF at hydrophobic core regions. Importantly, mutation V68βA in the six-residue mutant provides more space to accommodate the bulky side chain of cephalosporin C, which could help in designing cephalosporin C acylase mutants with higher activities and the practical one-step enzymatic route to prepare 7-aminocephalosporanic acid at industrial-scale levels.






Similar content being viewed by others
References
Aramori I, Fukagawa M, Tsumura M, Iwami M, Isogai T, Ono H, Ishitani Y, Kojo H, Kohsaka M, Ueda Y, Imanaka H (1991) Cloning and nucleotide sequencing of new glutaryl 7-ACA and cephalosporin C acylase genes from Pseudomonas strains. J Ferment Bioeng 72(4):232–243. doi:10.1016/0922-338X(91)90155-A
Bednar D, Beerens K, Sebestova E, Bendl J, Khare S, Chaloupkova R, Prokop Z, Brezovsky J, Baker D, Damborsky J (2015) Fireprot: energy- and evolution-based computational design of thermostable multiple-point mutants. PLoS Comput Biol 11(11):e1004556. doi:10.1371/journal.pcbi.1004556
Bjelic S, Nivón LG, Çelebi-Ölçüm N, Kiss G, Rosewall CF, Lovick HM, Ingalls EL, Gallaher JL, Seetharaman J, Lew S, Montelione GT, Hunt JF, Michael FE, Houk KN, Baker D (2013) Computational design of enone-binding proteins with catalytic activity for the Morita–Baylis–Hillman reaction. ACS Chem Biol 8(4):749–757. doi:10.1021/cb3006227
Bloom JD, Labthavikul ST, Otey CR, Arnold FH (2006) Protein stability promotes evolvability. Proc Natl Acad Sci U S A 103(15):5869–5874. doi:10.1073/pnas.0510098103
Bolon DN, Mayo SL (2001) Enzyme-like proteins by computational design. Proc Natl Acad Sci U S A 98(25):14274–14279. doi:10.1073/pnas.251555398
Borgo B, Havranek JJ (2012) Automated selection of stabilizing mutations in designed and natural proteins. Proc Natl Acad Sci U S A 109(5):1494–1499. doi:10.1073/pnas.1115172109
Bornscheuer U, Huisman G, Kazlauskas R, Lutz S, Moore J, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485(7397):185–194. doi:10.1038/nature11117
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254. doi:10.1016/0003-2697(76)90527-3
Conti G, Pollegioni L, Molla G, Rosini E (2014) Strategic manipulation of an industrial biocatalyst—evolution of a cephalosporin C acylase. FEBS J 281(10):2443–2455. doi:10.1111/febs.12798
Diaz JE, Lin C, Kunishiro K, Feld BK, Avrantinis SK, Bronson J, Greaves J, Saven JG, Weiss GA (2011) Computational design and selections for an engineered, thermostable terpene synthase. Protein Sci 20(9):1597–1606. doi:10.1002/pro.691
Golden E, Paterson R, Tie WJ, Anandan A, Flematti G, Molla G, Rosini E, Pollegioni L, Vrielink A (2013) Structure of a class III engineered cephalosporin acylase: comparisons with class I acylase and implications for differences in substrate specificity and catalytic activity. Biochem J 451(2):217–226. doi:10.1042/BJ20121715
Gordon SR, Stanley EJ, Wolf S, Toland A, Wu SJ, Hadidi D, Mills JH, Baker D, Pultz IS, Siegel JB (2012) Computational design of an α-gliadin peptidase. J Am Chem Soc 134(50):20513–20520. doi:10.1021/ja3094795
Gray KA, Richardson TH, Kretz K, Short JM, Bartnek F, Knowles R, Kan L, Swanson PE, Robertson DE (2001) Rapid evolution of reversible denaturation and elevated melting temperature in a microbial haloalkane dehalogenase. Adv Synth Catal 343(6–7):607–617. doi:10.1002/1615-4169(200108)343:6/7<607::AID-ADSC607>3.0.CO;2-M
Huang X, Han K, Zhu Y (2013) Systematic optimization model and algorithm for binding sequence selection in computational enzyme design. Protein Sci 22(7):929–941. doi:10.1002/pro.2275
Huang X, Xue J, Lin M, Zhu Y (2016) Use of an improved matching algorithm to select scaffolds for enzyme design based on a complex active site model. PLoS One 11(5):e0156559. doi:10.1371/journal.pone.0156559
Ishii Y, Saito Y, Fujimura T, Sasaki H, Noguchi Y, Yamada H, Niwa M, Shimomura K (1995) High-level production, chemical modification and site-directed mutagenesis of a cephalosporin C acylase from Pseudomonas strain N176. Eur J Biochem 230(2):773–778. doi:10.1111/j.1432-1033.1995.0773h.x
Jiang L, Althoff EA, Clemente FR, Doyle L, Röthlisberger D, Zanghellini A, Gallaher JL, Betker JL, Tanaka F, Barbas CF, Hilvert D, Houk KN, Stoddard BL, Baker D (2008) De novo computational design of retro-aldol enzymes. Science 319(5868):1387–1391. doi:10.1126/science.1152692
Johannes TW, Woodyer RD, Zhao H (2005) Directed evolution of a thermostable phosphite dehydrogenase for NAD(P)H regeneration. Appl Environ Microbiol 71(10):5728–5734. doi:10.1128/aem.71.10.5728-5734.2005
Khare SD, Kipnis Y, Greisen P Jr, Takeuchi R, Ashani Y, Goldsmith M, Song Y, Gallaher JL, Silman I, Leader H, Sussman JL, Stoddard BL, Tawfik DS, Baker D (2012) Computational redesign of a mononuclear zinc metalloenzyme for organophosphate hydrolysis. Nat Chem Biol 8(3):294–300. doi:10.1038/nchembio.777
Kim Y, Yoon K, Khang Y, Turley S, Hol WGJ (2000) The 2.0 Å crystal structure of cephalosporin acylase. Structure 8(10):1059–1068. doi:10.1016/S0969-2126(00)00505-0
Korkegian A, Black ME, Baker D, Stoddard BL (2005) Computational thermostabilization of an enzyme. Science 308(5723):857–860. doi:10.1126/science.1107387
Lassila JK, Keeffe JR, Kast P, Mayo SL (2007) Exhaustive mutagenesis of six secondary active-site residues in Escherichia coli chorismate mutase shows the importance of hydrophobic side chains and a helix N-capping position for stability and catalysis. Biochemistry 46(23):6883–6891. doi:10.1021/bi700215x
Lei Y, Luo W, Zhu Y (2011) A matching algorithm for catalytic residue site selection in computational enzyme design. Protein Sci 20(9):1566–1575. doi:10.1002/pro.685
Lejon S, Ellis J, Valegård K (2008) The last step in cephalosporin C formation revealed: crystal structures of deacetylcephalosporin C acetyltransferase from Acremonium chrysogenum in complexes with reaction intermediates. J Mol Biol 377(3):935–944. doi:10.1016/j.jmb.2008.01.047
Li Q, Huang X, Zhu Y (2014) Evaluation of active designs of cephalosporin C acylase by molecular dynamics simulation and molecular docking. J Mol Model 20(7):1–12. doi:10.1007/s00894-014-2314-5
MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102(18):3586–3616. doi:10.1021/jp973084f
Malakauskas SM, Mayo SL (1998) Design, structure and stability of a hyperthermophilic protein variant. Nat Struct Mol Biol 5(6):470–475. doi:10.1038/nsb0698-470
Murphy PM, Bolduc JM, Gallaher JL, Stoddard BL, Baker D (2009) Alteration of enzyme specificity by computational loop remodeling and design. Proc Natl Acad Sci U S A 106(23):9215–9220. doi:10.1073/pnas.0811070106
Oelschlaeger P, Schmid RD, Pleiss J (2003) Modeling domino effects in enzymes: molecular basis of the substrate specificity of the bacterial metallo-β-lactamases IMP-1 and IMP-6. Biochemistry 42(30):8945–8956. doi:10.1021/bi0300332
Oh B, Kim M, Yoon J, Chung K, Shin Y, Lee D, Kim Y (2003) Deacylation activity of cephalosporin acylase to cephalosporin C is improved by changing the side-chain conformations of active-site residues. Biochem Biophys Res Commun 310(1):19–27. doi:10.1016/j.bbrc.2003.08.110
Ollikainen N, de Jong RM, Kortemme T (2015) Coupling protein side-chain and backbone flexibility improves the re-design of protein-ligand specificity. PLoS Comput Biol 11(9):e1004335. doi:10.1371/journal.pcbi.1004335
Palackal N, Brennan Y, Callen WN, Dupree P, Frey G, Goubet F, Hazlewood GP, Healey S, Kang YE, Kretz KA, Lee E, Tan X, Tomlinson GL, Verruto J, Wong VWK, Mathur EJ, Short JM, Robertson DE, Steer BA (2004) An evolutionary route to xylanase process fitness. Protein Sci 13(2):494–503. doi:10.1110/ps.03333504
Pollegioni L, Lorenzi S, Rosini E, Marcone GL, Molla G, Verga R, Cabri W, Pilone MS (2005) Evolution of an acylase active on cephalosporin C. Protein Sci 14(12):3064–3076. doi:10.1110/ps.051671705
Pollegioni L, Rosini E, Molla G (2013) Cephalosporin C acylase: dream and(/or) reality. Appl Microbiol Biotechnol 97(6):2341–2355. doi:10.1007/s00253-013-4741-0
Richter F, Blomberg R, Khare SD, Kiss G, Kuzin AP, Smith AJT, Gallaher J, Pianowski Z, Helgeson RC, Grjasnow A, Xiao R, Seetharaman J, Su M, Vorobiev S, Lew S, Forouhar F, Kornhaber GJ, Hunt JF, Montelione GT, Tong L, Houk KN, Hilvert D, Baker D (2012) Computational design of catalytic dyads and oxyanion holes for ester hydrolysis. J Am Chem Soc 134(39):16197–16206. doi:10.1021/ja3037367
Röthlisberger D, Khersonsky O, Wollacott AM, Jiang L, DeChancie J, Betker J, Gallaher JL, Althoff EA, Zanghellini A, Dym O, Albeck S, Houk KN, Tawfik DS, Baker D (2008) Kemp elimination catalysts by computational enzyme design. Nature 453(7192):190–195. doi:10.1038/nature06879
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts M, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature 409(6817):258–268. doi:10.1038/35051736
Shen MY, Sali A (2006) Statistical potential for assessment and prediction of protein structures. Protein Sci 15(11):2507–2524. doi:10.1110/ps.062416606
Siegel JB, Smith AL, Poust S, Wargacki AJ, Bar-Even A, Louw C, Shen BW, Eiben CB, Tran HM, Noor E, Gallaher JL, Bale J, Yoshikuni Y, Gelb MH, Keasling JD, Stoddard BL, Lidstrom ME, Baker D (2015) Computational protein design enables a novel one-carbon assimilation pathway. Proc Natl Acad Sci U S A 112(12):3704–3709. doi:10.1073/pnas.1500545112
Siegel JB, Zanghellini A, Lovick HM, Kiss G, Lambert AR, Clair JLS, Gallaher JL, Hilvert D, Gelb MH, Stoddard BL, Houk KN, Michael FE, Baker D (2010) Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction. Science 329(5989):309–313. doi:10.1126/science.1190239
Smith CA, Kortemme T (2008) Backrub-like backbone simulation recapitulates natural protein conformational variability and improves mutant side-chain prediction. J Mol Biol 380(4):742–756. doi:10.1016/j.jmb.2008.05.023
Tian Y, Huang X, Zhu Y (2015) Computational design of enzyme–ligand binding using a combined energy function and deterministic sequence optimization algorithm. J Mol Model 21(8):1–14. doi:10.1007/s00894-015-2742-x
Volpato GC, Rodrigues R, Fernandez-Lafuente R (2010) Use of enzymes in the production of semi-synthetic penicillins and cephalosporins: drawbacks and perspectives. Curr Med Chem 17(32):3855–3873. doi:10.2174/092986710793205435
Walsh C (2001) Enabling the chemistry of life. Nature 409(6817):226–231. doi:10.1038/35051697
Wijma HJ, Floor RJ, Bjelic S, Marrink SJ, Baker D, Janssen DB (2015) Enantioselective enzymes by computational design and in silico screening. Angew Chem Int Ed 54(12):3726–3730. doi:10.1002/anie.201411415
Wijma HJ, Floor RJ, Janssen DB (2013) Structure- and sequence-analysis inspired engineering of proteins for enhanced thermostability. Curr Opin Struc Biol 23(4):588–594. doi:10.1016/j.sbi.2013.04.008
Wijma HJ, Floor RJ, Jekel PA, Baker D, Marrink SJ, Janssen DB (2014) Computationally designed libraries for rapid enzyme stabilization. Protein Eng Des Sel 27(2):49–58. doi:10.1093/protein/gzt061
Xiang Z, Honig B (2001) Extending the accuracy limits of prediction for side-chain conformations. J Mol Biol 311(2):421–430. doi:10.1006/jmbi.2001.4865
Zhu Y (2007) Mixed-integer linear programming algorithm for a computational protein design problem. Ind Eng Chem Res 46(3):839–845. doi:10.1021/ie0605985
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Numbers 21276136, 21476123), and the National High Technology Research and Development (863) Program of China (Grant Number 2012AA021204).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 150 kb)
Rights and permissions
About this article
Cite this article
Tian, Y., Huang, X., Li, Q. et al. Computational design of variants for cephalosporin C acylase from Pseudomonas strain N176 with improved stability and activity. Appl Microbiol Biotechnol 101, 621–632 (2017). https://doi.org/10.1007/s00253-016-7796-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7796-x