Abstract
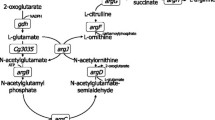
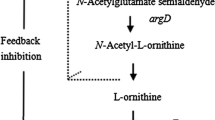
l-arginine is a semi-essential amino acid with application in cosmetic, pharmaceutical, and food industries. Metabolic engineering strategies have been applied for overproduction of l-arginine by Corynebacterium glutamicum. LysE was the only known l-arginine exporter of this bacterium. However, an l-arginine-producing strain carrying a deletion of lysE still accumulated about 10 mM l-arginine in the growth medium. Overexpression of the putative putrescine and cadaverine export permease gene cgmA was shown to compensate for the lack of lysE with regard to l-arginine export. Moreover, plasmid-borne overexpression of cgmA rescued the toxic effect caused by feeding of the dipeptide Arg-Ala to lysE-deficient C. glutamicum and argO-deficient Escherichia coli strains. Deletion of the repressor gene cgmR improved l-arginine titers by 5 %. Production of l-lysine and l-citrulline was not affected by cgmA overexpression. Taken together, CgmA may function as an export system not only for the diamine putrescine and cadaverine but also for l-arginine. The major export system for l-lysine and l-arginine LysE may also play a role in l-citrulline export since production of l-citrulline was reduced when lysE was deleted and improved by 45 % when lysE was overproduced.





Similar content being viewed by others
References
Abe S, Takayarna K, Kinoshita S (1967) Taxonomical studies on glutamicum acid producing bacteria. J Gen Appl Microbiol 13:279–301
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006 0008. doi:10.1038/msb4100050
Baumgart M, Unthan S, Ruckert C, Sivalingam J, Grunberger A, Kalinowski J, Bott M, Noack S, Frunzke J (2013) Construction of a prophage-free variant of Corynebacterium glutamicum ATCC 13032 for use as a platform strain for basic research and industrial biotechnology. Appl Environ Microbiol 79:6006–6015. doi:10.1128/AEM.01634-13
Bellmann A, Vrljic M, Patek M, Sahm H, Kramer R, Eggeling L (2001) Expression control and specificity of the basic amino acid exporter LysE of Corynebacterium glutamicum. Microbiology 147:1765–1774. doi:10.1099/00221287-147-7-1765
Broer S, Eggeling L, Kramer R (1993) Strains of Corynebacterium glutamicum with different lysine productivities may have different lysine excretion systems. Appl Environ Microbiol 59:316–321
Broer S, Krämer R (1991) Lysine excretion by Corynebacterium glutamicum. 1. Identification of a specific secretion carrier system. Eur J Biochem 202:131–135
Burkovski A, Krämer R (2002) Bacterial amino acid transport proteins: occurrence, functions, and significance for biotechnological applications. Appl Microbiol Biotechnol 58:265–274. doi:10.1007/s00253-001-0869-4
Busch W, Saier MH Jr, International Union of B, Molecular B (2004) The IUBMB-endorsed transporter classification system. Mol Biotechnol 27:253–262
Diesveld R, Tietze N, Furst O, Reth A, Bathe B, Sahm H, Eggeling L (2009) Activity of exporters of Escherichia coli in Corynebacterium glutamicum, and their use to increase L-threonine production. J Mol Microbiol Biotechnol 16:198–207. doi:10.1159/000142530
Eberhardt D, Jensen JV, Wendisch VF (2014) L-citrulline production by metabolically engineered Corynebacterium glutamicum from glucose and alternative carbon sources. AMB Express 4:85. doi:10.1186/s13568-014-0085-0
Eggeling L, Bott M (2005) Handbook of Corynebacterium glutamicum. CRC Press
Eggeling L, Sahm H (2001) The cell wall barrier of Corynebacterium glutamicum and amino acid efflux. J Biosci Bioeng 92:201–213. doi:10.1263/Jbb.92.201
Erdmann A, Weil B, Krämer R (1993) Lysine secretion by wild-type Corynebacterium glutamicum triggered by dipeptide uptake. J Gen Microbiol 139:3115–3122
Fluman N, Bibi E (2009) Bacterial multidrug transport through the lens of the major facilitator superfamily. Biochim Biophys Acta 1794:738–747. doi:10.1016/j.bbapap.2008.11.020
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hoischen C, Krämer R (1989) Evidence for an efflux carrier system involved in the secretion of glutamate by Corynebacterium glutamicum. Arch Microbiol 151:342–347
Ikeda M, Mitsuhashi S, Tanaka K, Hayashi M (2009) Reengineering of a Corynebacterium glutamicum L-arginine and L-citrulline producer. Appl Environ Microbiol 75:1635–1641
Itou H, Okada U, Suzuki H, Yao M, Wachi M, Watanabe N, Tanaka I (2005) The CGL2612 protein from Corynebacterium glutamicum is a drug resistance-related transcriptional repressor: structural and functional analysis of a newly identified transcription factor from genomic DNA analysis. J Biol Chem 280:38711–38719. doi:10.1074/jbc.M505999200
Jensen JV, Eberhardt D, Wendisch VF (2015) Modular pathway engineering of Corynebacterium glutamicum for production of the glutamate-derived compounds ornithine, proline, putrescine, citrulline, and arginine. J Biotechnol 214:85–94
Jensen JV, Wendisch VF (2013) Ornithine cyclodeaminase-based proline production by Corynebacterium glutamicum. Microb Cell Factories 12:63
Keilhauer C, Eggeling L, Sahm H (1993) Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J Bacteriol 175:5595–5603
Kennerknecht N, Sahm H, Yen MR, Patek M, Saier MH Jr, Eggeling L (2002) Export of L-isoleucine from Corynebacterium glutamicum: a two-gene-encoded member of a new translocator family. J Bacteriol 184:3947–3956
Kimura E (2003) Metabolic engineering of glutamate production. Adv Biochem Eng Biotechnol 79:37–57
Kind S, Kreye S, Wittmann C (2011) Metabolic engineering of cellular transport for overproduction of the platform chemical 1,5-diaminopentane in Corynebacterium glutamicum. Metab Eng 13:617–627
Law CJ, Maloney PC, Wang DN (2008) Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol 62:289–305. doi:10.1146/annurev.micro.61.080706.093329
Lessmeier L, Pfeifenschneider J, Carnicer M, Heux S, Portais JC, Wendisch VF (2015) Production of carbon-13-labeled cadaverine by engineered Corynebacterium glutamicum using carbon-13-labeled methanol as co-substrate. Appl Microbiol Biotechnol 99:10163–10176. doi:10.1007/s00253-015-6906-5
Marin K, Krämer R (2007) Amino acid transport systems in biotechnologically relevant bacteria. In: Wendisch V (ed) Amino acid biosynthesis—pathways, regulation and metabolic engineering, vol 4. Microbiology Monographs, Springer. doi:10.1007/978-3-540-48596-4
Nakamura J, Hirano S, Ito H, Wachi M (2007) Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce L-glutamic acid production. Appl Environ Microbiol 73:4491–4498. doi:10.1128/AEM.02446-06
Nandineni MR, Gowrishankar J (2004) Evidence for an arginine exporter encoded by yggA (argO) that is regulated by the LysR-type transcriptional regulator ArgP in Escherichia coli. J Bacteriol 186:3539–3546. doi:10.1128/JB.186.11.3539-3546.2004
Nguyen AQ, Schneider J, Wendisch VF (2015) Elimination of polyamine N-acetylation and regulatory engineering improved putrescine production by Corynebacterium glutamicum. J Biotechnol 201:75–85. doi:10.1016/j.jbiotec.2014.10.035
Nikaido H (1994) Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382–388
Palmieri L, Berns D, Krämer R, Eikmanns M (1996) Threonine diffusion and threonine transport in Corynebacterium glutamicum and their role in threonine production. Arch Microbiol 165:48–54
Park SH, Kim HU, Kim TY, Park JS, Kim SS, Lee SY (2014) Metabolic engineering of Corynebacterium glutamicum for L-arginine production. Nat Commun 5:4618
Peter H, Weil B, Burkovski A, Kramer R, Morbach S (1998) Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing, and characterization of the proline/ectoine uptake system, ProP, and the ectoine/proline/glycine betaine carrier, EctP. J Bacteriol 180:6005–6012
Peters-Wendisch P, Gotker S, Heider SA, Komati Reddy G, Nguyen AQ, Stansen KC, Wendisch VF (2014) Engineering biotin prototrophic Corynebacterium glutamicum strains for amino acid, diamine and carotenoid production. J Biotechnol. doi:10.1016/j.jbiotec.2014.01.023
Peters-Wendisch PG, Schiel B, Wendisch VF, Katsoulidis E, Mockel B, Sahm H, Eikmanns BJ (2001) Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J Mol Microbiol Biotechnol 3:295–300
Saier MH Jr (2000) Families of transmembrane transporters selective for amino acids and their derivatives. Microbiology 146(Pt 8):1775–1795. doi:10.1099/00221287-146-8-1775
Saier MH Jr, Beatty JT, Goffeau A, Harley KT, Heijne WH, Huang SC, Jack DL, Jahn PS, Lew K, Liu J, Pao SS, Paulsen IT, Tseng TT, Virk PS (1999) The major facilitator superfamily. J Mol Microbiol Biotechnol 1:257–279
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schäfer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Schneider J, Niermann K, Wendisch VF (2011) Production of the amino acids l-glutamate, l-lysine, l-ornithine and l-arginine from arabinose by recombinant Corynebacterium glutamicum. J Biotechnol 154:191–198. doi:10.1016/j.jbiotec.2010.07.009
Seryoung K, Yoneyama H (2013) Amino acid exporter: a tool for the next-generation microbial fermentation. J Biotechnol Biomater 3:118
Simic P, Sahm H, Eggeling L (2001) L-threonine export: use of peptides to identify a new translocator from Corynebacterium glutamicum. J Bacteriol 183:5317–5324
Simic P, Willuhn J, Sahm H, Eggeling L (2002) Identification of glyA (encoding serine hydroxymethyltransferase) and its use together with the exporter ThrE to increase L-threonine accumulation by Corynebacterium glutamicum. Appl Environ Microbiol 68:3321–3327
Stansen C, Uy D, Delaunay S, Eggeling L, Goergen JL, Wendisch VF (2005) Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl Environ Microbiol 71:5920–5928
Trötschel C, Deutenberg D, Bathe B, Burkovski A, Kramer R (2005) Characterization of methionine export in Corynebacterium glutamicum. J Bacteriol 187:3786–3794. doi:10.1128/JB.187.11.3786-3794.2005
van der Rest ME, Lange C, Molenaar D (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl Microbiol Biotechnol 52:541–545
Vrljic M, Sahm H, Eggeling L (1996) A new type of transporter with a new type of cellular function: L-lysine export from Corynebacterium glutamicum. Mol Microbiol 22:815–826
Wendisch VF (2014) Microbial production of amino acids and derived chemicals: synthetic biology approaches to strain development. Curr Opin Biotechnol 30:51–58. doi:10.1016/j.copbio.2014.05.004
Xu M, Rao Z, Yang J, Dou W, Xu Z (2013) The effect of a LysE exporter overexpression on L-arginine production in Corynebacterium crenatum. Curr Microbiol 67:271–278. doi:10.1007/s00284-013-0358-x
Yamada S, Awano N, Inubushi K, Maeda E, Nakamori S, Nishino K, Yamaguchi A, Takagi H (2006) Effect of drug transporter genes on cysteine export and overproduction in Escherichia coli. Appl Environ Microbiol 72:4735–4742. doi:10.1128/Aem.02507-05
Zittrich S, Krämer R (1994) Quantitative discrimination of carrier-mediated excretion of isoleucine from uptake and diffusion in Corynebacterium glutamicum. J Bacteriol 176:6892–6899
Acknowledgments
We wish to thank Linea Muhsal for her contribution and preliminary experimental results and Lothar Eggeling for providing the plasmid pk18mobsacBΔlysEG. João M. P. Jorge and Fernando Pérez-García acknowledge PhD fellowships from the CLIB2021 graduate cluster at Bielefeld University. Hironori Taniguchi acknowledges support by a PhD fellowship from the DAAD. This work was supported in part by grant KF2969003SB2 from BMWi in the ZIM program.
Author’s contributions
J.M.P.J., D.L., H.T., and V.F.W. designed the study. J.M.P.J., F.P.G., and D.L. performed the experiments. All authors analyzed the data and drafted the manuscript. V.F.W. finalized the manuscript. All authors read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research performed did not involve human participants and/or animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Dorit Lubitz and João M. P. Jorge contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lubitz, D., Jorge, J.M.P., Pérez-García, F. et al. Roles of export genes cgmA and lysE for the production of l-arginine and l-citrulline by Corynebacterium glutamicum . Appl Microbiol Biotechnol 100, 8465–8474 (2016). https://doi.org/10.1007/s00253-016-7695-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7695-1