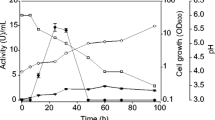
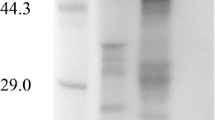
Abstract
Basidiomycetous yeast Xanthophyllomyces dendrorhous expresses an α-glucosidase with strong transglycosylation activity producing prebiotic sugars such as panose and an unusual tetrasaccharides mixture including α–(1–6) bonds as major products, which makes it of biotechnological interest. Initial analysis pointed to a homodimeric protein of 60 kDa subunit as responsible for this activity. In this study, the gene Xd-AlphaGlu was characterized. The 4131-bp-long gene is interrupted by 13 short introns and encodes a protein of 990 amino acids (Xd-AlphaGlu). The N-terminal sequence of the previously detected 60 kDa protein resides in this larger protein at residues 583–602. Functionality of the gene was proved in Saccharomyces cerevisiae, which produced a protein of about 130 kDa containing Xd-AlphaGlu sequences. All properties of the heterologously expressed protein, including thermal and pH profiles, activity on different substrates, and ability to produce prebiotic sugars were similar to that of the α-glucosidase produced in X. dendrorhous. No activity was detected in S. cerevisiae containing exclusively the 1256-bp from gene Xd-AlphaGlu that would encode synthesis of the 60 kDa protein previously detected. Data were compatible with an active monomeric α-glucosidase of 990 amino acids and an inactive hydrolysis product of 60 kDa. Protein Xd-AlphaGlu contained most of the elements characteristic of α-glucosidases included in the glycoside hydrolases family GH31 and its structural model based on the homologous human maltase-glucoamylase was obtained. Remarkably, the Xd-AlphaGlu C-terminal domain presents an unusually long 115-residue insertion that could be involved in this enzyme’s activity against long-size substrates such as maltoheptaose and soluble starch.







Similar content being viewed by others
References
Arnold K, Bordoli L, Koop J, Schwede T (2006) The Swiss-model workspace: a web-based environment for protein structure homology modeling. Bioinformatics 22:195–201. doi:10.1093/bioinformatics/bti770
Baeza M, Alcaíno J, Barahona S, Sepulveda D, Cifuentes V (2015) Codon usage and codon context bias in Xanthophyllomyces dendrorhous. BMC Genomics 16:293. doi:10.1186/s12864-015-1493-5
Bang ML, Villadsen I, Sandal T (1999) Cloning and characterization of an endo-β-1,3(4)glucanase and an aspartic protease from Phaffia rhodozyma CBS 6938. Appl Microbiol Biotechnol 51:215–222. doi:10.1007/s002530051384
Bon E, Casaregola S, Blandin G, Llorente B, Neuveglise C, Münsterkotter M, Güldener U, Mewes HW, Van Helden J, Dujon B, Gaillardin C (2003) Molecular evolution of eukaryotic genomes: hemiascomycetous yeast spliceosomal introns. Nucleic Acids Res 31:1121–1135. doi:10.1093/nar/gkg213
Burke D, Dawson D, Stearns T (2000) Methods in yeast genetics: A Cold Spring Harbour Laboratory course manual. Cold Spring Harbor Laboratory Press, Plainview, N.Y
Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T (2005) MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21:2933–42. doi:10.1093/bioinformatics/bti473
Chiba S (1997) Molecular mechanism in α-glucosidase and glucoamylase. Biosci Biotechnol Biochem 61:1233-1239. doi.org/10.1271/bbb.61.1233
Fernández-Arrojo L, Marín D, Gómez de Segura A, Linde D, Alcalde M, Gutiérrez-Alonso P, Ghazi I, Plou FJ, Fernández-Lobato M, Ballesteros A (2007) Transformation of maltose into prebiotic isomaltooligosaccharides by a novel α-glucosidase from Xantophyllomyces dendrorhous. Process Biochem 42:1530–1536. doi:10.1016/j.procbio.2007.08.007
Goffin D, Delzenne N, Blecker C, Hanon E, Deroanne C, Paquot M (2011) Will isomalto-oligosaccharides, a well-established functional food in Asia, break through the European and American market? The status of knowledge on these prebiotics. Crit Rev Food Sci and Nutr 51:394–409. doi:10.1080/10408391003628955
Henrissat B, Davies G (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637-644.
Hermans MM, Kroos MA, van Beeumen J, Oostra BA, Reuser AJ (1991) Human lysosomal α-glucosidase. Characterization of the catalytic site. J Biol Chem 266:13507–13512
Janecek S, Svensson B, MacGregor EA (2014) α-Amylase: an enzyme specificity found in various families of glycoside hydrolases. Cell Mol Life Sci 71:1149–1170. doi:10.1007/s00018-013-1388-z
Kato N, Suyama S, Shirokane M, Kato M, Kobayashi T, Tsukagoshi N (2002) Novel α-glucosidase from Aspergillus nidulans with strong transglycosylation activity. Appl Environ Microbiol 68:1250–1256. doi:10.1128/AEM.68.3.1250-1256.2002
Kofod LV, Kauppinen S, Christgau S, Andersen LN, Heldt-Hansen HP, Dörreich K, Dalbøge H (1994) Cloning and characterization of two structurally and functionally divergent rhamnogalacturonases from Aspergillus aculeatus. J Biol Chem 269:29182–29189
Kupfer DM, Drabenstot SD, Buchanan KL, Lai H, Zhu H, Dyer DW, Roe BA, Murphy JW (2004) Introns and splicing elements of five diverse fungi. Eukaryot Cell 3:1088-1100. doi:10.1128/EC.3.5.1088-1100
Linde D, Macias I, Fernández-Arrojo L, Plou FJ, Jiménez A, Fernández-Lobato M (2009) Molecular and biochemical characterization of a β-fructofuranosidase from Xanthophyllomyces dendrorhous. Appl Environ Microbiol 75:1065–1073. doi:10.1128/AEM.02061-08
Loto I, Gutiérrez MS, Barahona S, Sepúlveda D, Martínez-Moya P, Baeza M, Cifuentes V, Alcaíno J (2012) Enhancement of carotenoid production by disrupting the C22-sterol desaturase gene (CYP61) in Xanthophyllomyces dendrorhous. BMC Microbiol 12:235. doi:10.1186/1471-2180-12-2351471-2180-12-235
Lovering AL, Lee SS, Kim YW, Withers SG, Strynadka NC (2005) Mechanistic and structural analysis of a family 31 α-glycosidase and its glycosyl-enzyme intermediate. J Biol Chem 280:2105-2115. doi:10.1074/jbc.M410468200
Marín D, Linde D, Fernández Lobato M (2006) Purification and biochemical characterization of an α-glucosidase from Xanthophyllomyces dendrorhous. Yeast 23:117–125. doi:10.1002/yea.1345S0378-1119(11)00511-7
Melo EB, Gomes AS, Carvalho I (2006) α- and β-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron 62:10277–10302. doi:10.1016/j.tet.2006.08.055
Mohan S, Eskandari R, Pinto BM (2014) Naturally occurring sulfonium-ion glucosidase inhibitors and their derivatives: a promising class of potential antidiabetic agents. Acc Chem Res 47:211–225. doi:10.1021/ar400132g
Pal K, Kumar S, Sharma S, Garg SK, Alam MS, Xu HE, Agrawal P, Swaminathan K (2010) Crystal structure of full-length Mycobacterium tuberculosis H37Rv glycogen branching enzyme: insights of N-terminal β-sandwich in substrate specificity and enzymatic activity. J Biol Chem 285:20897–20903. doi:10.1074/jbc.M110.121707M110.121707
Pan YC, Lee WC (2005) Production of high-purity isomalto-oligosaccharides syrup by the enzymatic conversion of transglucosidase and fermentation of yeast cells. Biotechnol Bioeng 89:797–804. doi:10.1002/bit.20402
Raben N, Plotz P, Byrne BJ (2002) Acid α-glucosidase deficiency (glycogenosis type II, Pompe disease). Curr Mol Med 2:145–166. doi:10.2174/1566524024605789#sthash.KQI1znTD.dpuf
Rehm J, Willmitzer L, Heyer AG (1998) Production of 1-kestose in transgenic yeast expressing a fructosyltransferase from Aspergillus foetidus. J Bacteriol 180:1305–1310
Ren LM, Qin XH, Cao XF, Wang LL, Bai F, Bai G, Shen Y (2011) Structural insight into substrate specificity of human intestinal maltase-glucoamylase. Protein Cell 2:827–836. doi:10.1007/s13238-011-1105-3
Rosenberg AH, Goldman E, Dunn JJ, Studier FW, Zubay G (1993) Effects of consecutive AGG codons on translation in Escherichia coli, demonstrated with a versatile codon test system. J Bacteriol 175:716–722
Sharma R, Gassel S, Steiger S, Xia X, Bauer R, Sandmann G, Thines M (2015) The genome of the basal agaricomycete Xanthophyllomyces dendrorhous provides insights into the organziation of its acetyl-CoA derived pathways and the evolution of Agaricomycotina. BMC Genomics 16:233. doi:10.1186/s12864-015-1380-0
Shen X, Saburi W, Gai ZQ, Komoda K, Yu J, Ojima-Kato T, Kido Y, Matsui H, Mori H, Yao M (2014) Crystallization and preliminary x-ray crystallographic analysis of α-glucosidase HaG from Halomonas sp. strain H11. Acta Crystallogr F Struct Biol Commun 70:464–466. doi:10.1107/S2053230X14001940S2053230X14001940
Shirai T, Hung VS, Morinaka K, Kobayashi T, Ito S (2008) Crystal structure of GH13 α-glucosidase GSJ from one of the deepest sea bacteria. Proteins 73:126–133. doi:10.1002/prot.22044
Sim L, Quezada-Calvillo R, Sterchi EE, Nichols BL, Rose DR (2008) Human intestinal maltase–glucoamylase: crystal structure of the N-terminal catalytic subunit and basis of inhibition and substrate specificity. J Mol Biol 375:782–792. doi:10.1016/j.jmb.2007.10.069
Tagami T, Yamashita K, Okuyama M, Mori H, Yao M, Kimura A (2013) Molecular basis for the recognition of long-chain substrates by plant α-glucosidases. J Biol Chem 288:19296–19303. doi:10.1074/jbc.M113.465211jbc.M113.465211
Tagami T, Yamashita K, Okuyama M, Mori H, Yao M, Kimura A (2015) Structural advantage of sugar beet α-glucosidase to stabilize the Michaelis complex with long-chain substrate. J Biol Chem 290:1796–1803. doi:10.1074/jbc.M114.606939M114.606939
Wang YH, Jiang Y, Duan ZY, Shao WL, Li HZ (2009) Expression and characterization of an α-glucosidase from Thermoanaerobacter ethanolicus JW200 with potential for industrial application. Biologia 64:1053–1057. doi:10.2478/s11756-009-0197-1
Wu KY, Huang SH, Ding S, Zhang YK, Chen GG, Liang ZQ (2010) Expression, purification and characterization of recombinant α-glucosidase in Pichia pastoris. Folia Microbiol 66:582–587. doi:10.1007/s12223-010-0093-7
Acknowledgments
Projects BIO2013-48779-C4-1/-3/-4 from the Spanish Ministry of Economy and Competitiveness supported this research. We thank Fundación Ramón Areces for the institutional grant to the Centro de Biología Molecular Severo Ochoa. M.G.P. was supported by a Spanish FPU fellowship from the Ministry of Economy and Competitiveness.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Spanish Ministry of Economy and Competitiveness (BIO2013-48779-C4-1/-3/-4).
Conflict of interests
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1
Table S1. Characteristics of the Xd-AlphaGlu introns. Figure S1. Positioning of peptide masses generated by the MALDI-TOF analysis of the protein Xd-AlphaGlu expressed in X. dendrorhous (a) and S. cerevisiae (b). The peptide m/z is indicated. (PDF 339 kb)
Rights and permissions
About this article
Cite this article
Gutiérrez-Alonso, P., Gimeno-Pérez, M., Ramírez-Escudero, M. et al. Molecular characterization and heterologous expression of a Xanthophyllomyces dendrorhous α-glucosidase with potential for prebiotics production. Appl Microbiol Biotechnol 100, 3125–3135 (2016). https://doi.org/10.1007/s00253-015-7171-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7171-3