Abstract
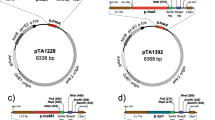
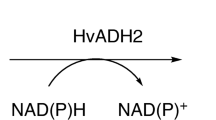
The success of biotechnological processes is based on the availability of efficient and highly specific biocatalysts, which can satisfy industrial demands. Extreme and remote environments like the deep brine pools of the Red Sea represent highly interesting habitats for the discovery of novel halophilic and thermophilic enzymes. Haloferax volcanii constitutes a suitable expression system for halophilic enzymes obtained from such brine pools. We developed a batch process for the cultivation of H. volcanii H1895 in controlled stirred-tank bioreactors utilising knockouts of components of the flagella assembly system. The standard medium Hv-YPC was supplemented to reach a higher cell density. Without protein expression, cell dry weight reaches 10 g L−1. Two halophilic alcohol dehydrogenases were expressed under the control of the tryptophanase promoter p.tna with 16.8 and 3.2 mg gCDW −1, respectively, at a maximum cell dry weight of 6.5 g L−1. Protein expression was induced by the addition of l-tryptophan. Investigation of various expression strategies leads to an optimised two-step induction protocol introducing 6 mM l-tryptophan at an OD650 of 0.4 followed by incubation for 16 h and a second induction step with 3 mM l-tryptophan followed by a final incubation time of 4 h. Compared with the uncontrolled shaker-flask cultivations used until date, dry cell mass concentrations were improved by a factor of more than 5 and cell-specific enzyme activities showed an up to 28-fold increased yield of the heterologous proteins.









Similar content being viewed by others
References
Albers SV, Pohlschroder M (2009) Diversity of archaeal type IV pilin-like structures. Extremophiles 13(3):403–10. doi:10.1007/s00792-009-0241-7
Allers T (2010) Overexpression and purification of halophilic proteins in Haloferax volcanii. Bioengineered Bugs 1(4):288–290. doi:10.1128/AEM.02670-09
Allers T, Ngo HP, Mevarech M, Lloyd RG (2004) Development of additional selectable markers for the halophilic Archaeon Haloferax volcanii based on the leuB and trpA genes. Appl Environ Microbiol 70(2):943–953. doi:10.1128/aem.70.2.943-953.2004
Allers T, Barak S, Liddell S, Wardell K, Mevarech M (2010) Improved strains and plasmid vectors for conditional overexpression of His-tagged proteins in Haloferax volcanii. Appl Environ Microbiol 76(6):1759–69. doi:10.1128/AEM.02670-09
Antunes A, Ngugi DK, Stingl U (2011) Microbiology of the Red Sea (and other) deep-sea anoxic brine lakes. Environ Microbiol Rep 3(4):416–33. doi:10.1111/j.1758-2229.2011.00264.x
Arends I, Sheldon RA, Hanefeld U (2007) Introduction: green chemistry and catalysis green chemistry and catalysis I. WILEY-VCH Verlag GmbH & Co, KGaA
Bertani G (1951) Studies on lysogenesis. J Bacteriol 62(3):293–300
Bitan-Banin G, Ortenberg R, Mevarech M (2003) Development of a gene knockout system for the halophilic archaeon Haloferax volcanii by use of the pyrE gene. J Bacteriol 185(3):772–778. doi:10.1128/jb.185.3.772-778.2003
Braesen C, Schoenheit P (2001) Mechanisms of acetate formation and acetate activation in halophilic archaea. Arch Microbiol 175(5):360–368. doi:10.1007/s002030100273
Cao Y, Liao L, Xu XW, Oren A, Wang C, Zhu XF, Wu M (2008) Characterization of alcohol dehydrogenase from the haloalkaliphilic archaeon Natronomonas pharaonis. Extremophiles 12(3):471–6. doi:10.1007/s00792-007-0133-7
Connaris H, Chaushuri JB, Danson MJ, Hough DW (1998a) Expression, reactivation and purification of enzymes from Haloferax volcanii in Escherichia coli. Biotechnol Bioeng 64(1):38–45
Connaris H, West MW, Hough DW, Danson MJ (1998b) Cloning and overexpression in Escherichia coli of the gene encoding citrate synthase from the hyperthermophilic Archaeon Sulfolobis solfataricus. Extremophiles 2:61–66
Danson MJ, Hough DW (1998) Structure, function and stability of enzymes from the Archaea. Trends Microbiol 6(8):307–314
Eder W, Jahnke LL, Schmidt M, Huber R (2001) Microbial diversity of the brine-seawater interface of the Kebrit Deep, Red Sea, studied via 16S rRNA gene sequences and cultivation methods. Appl Environ Microbiol 67(7):3077–85. doi:10.1128/AEM.67.7.3077-3085.2001
Eichler J (2001) Biotechnological uses of archaeal extremozymes. Biotechnol Adv 19
Elleuche S, Schroder C, Sahm K, Antranikian G (2014) Extremozymes—biocatalysts with unique properties from extremophilic microorganisms. Curr Opin Biotechnol 29:116–23. doi:10.1016/j.copbio.2014.04.003
Esquivel RN, Pohlschroder M (2014) A conserved type IV pilin signal peptide H-domain is critical for the post-translational regulation of flagella-dependent motility. Mol Microbiol 93(3):494–504. doi:10.1111/mmi.12673
Fairbanks G, Steck TS, Wallach DFH (1971) Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10(13):2606–2617
Frols S, Dyall-Smith M, Pfeifer F (2012) Biofilm formation by haloarchaea. Environ Microbiol 14(12):3159–74. doi:10.1111/j.1462-2920.2012.02895.x
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook, vol 10, Springer. University of Hertfordshire, Hatfield, pp 571–607
Ghosh A, Albers SV (2011) Assembly and function of the archaeal flagellum. Biochem Soc Trans 39(1):64–9. doi:10.1042/BST0390064
Gross B, Ronen N, Honigman S, Livne E (1999) Tryptophan toxicity—time and dose response in rats. Tryptophan, serotonin and melatonin. Adv Exp Med Biol 467:507–516
Grötzinger SW, Alam I, Ba Alawi W, Bajic VB, Stingl U, Eppinger J (2014) Mining a database of single amplified genomes from Red Sea brine pool extremophiles—improving reliability of gene function prediction using a profile and pattern matching algorithm (PPMA). Front Microbiol 5:134. doi:10.3389/fmicb.2014.00134
Hatti-Kaul R, Tornvall U, Gustafsson L, Borjesson P (2007) Industrial biotechnology for the production of bio-based chemicals—a cradle-to-grave perspective. Trends Biotechnol 25(3):119–24. doi:10.1016/j.tibtech.2007.01.001
Hollmann F, Arends IWCE, Holtmann D (2011) Enzymatic reductions for the chemist. Green Chem 13(9):2285. doi:10.1039/c1gc15424a
Holmes ML, Dyall-Smith M (2000) Sequence and expression of a halobacterial beta-galactosidase. Mol Microbiol 36(1):114–122
Holmes M, Scopes RK, Moritz R, Simpson RJ, Englert C, Pfeifer F, Dyall-Smith M (1996) Purification and analysis of an extremely halophilic beta-galactosidase from Haloferax alicantei. Biochem Biophys Acta 1337:276–286
Holmes M, Kamekura M, Lam W, Nuttall S, Woods WG, Jablonski P, Serrano J, Ngui K, Antón J, Allers T (2008) The Halohandbook, vol 7
Hough DW, Danson MJ (1999) Extremozymes. Curr Opin Chem Biol 3:39–46
Karan R, Capes M, DasSarma P, DasSarma S (2013) Cloning, overexpression, purification and characterization of a polyextremophilic beta-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi. BMC Biotechnol 13:3
Kennedy SP, Ng WV, Salzberg SL, Hood L, DasSarma S (2001) Understanding the adaptation of Halobacterium sp. NRC-1 to its extreme environment through computational analysis of its genome sequence. Genome Res 11(10):1641–50. doi:10.1101/gr.190201
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Large A, Stamme C, Lange C, Duan Z, Allers T, Soppa J, Lund PA (2007) Characterization of a tightly controlled promoter of the halophilic archaeon Haloferax volcanii and its use in the analysis of the essential cct1 gene. Mol Microbiol 66(5):1092–106. doi:10.1111/j.1365-2958.2007.05980.x
Lestini R, Laptenok SP, Kuhn J, Hink MA, Schanne-Klein MC, Liebl U, Myllykallio H (2013) Intracellular dynamics of archaeal FANCM homologue Hef in response to halted DNA replication. Nucleic Acids Res 41(22):10358–70. doi:10.1093/nar/gkt816
Liliensiek AK, Cassidy J, Gucciardo G, Whitely C, Paradisi F (2013) Heterologous overexpression, purification and characterisation of an alcohol dehydrogenase (ADH2) from Halobacterium sp. NRC-1. Mol Biotechnol 55(2):143–149 doi:10.1007/s12033-013-9666-4
Madern D, Ebel C, Zaccai G (2000) Halophilic adaption of enzymes. Extremophiles 4:91–98
Mevarech M, Frolow F, Gloss LM (2000) Halophilic enzymes: proteins with a grain of salt. Biophys Chem 86:155–164
Meyer H-P (2011) Sustainability and biotechnology. Org Process Res Dev 15:180–188
Mironescu M, Mironescu ID, Jascanu V, Posten C (2003) Influence of cultivation media on halobacteria I growth and biomass formation. ACTA Univ Cibiniensis 1(7):17–24
Mullakhanbhai M, Larsen H (1975) Halobacterium volcanii spec. nov., a Dead-Sea halobacterium with a moderate salt requirement. Arch Microbiol 104:207–214
Oren A (2008) Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst 4:2. doi:10.1186/1746-1448-4-2
Puskeiler R, Kaufmann K, Weuster-Botz D (2005) Development, parallelization, and automation of a gas-inducing milliliter-scale bioreactor for high-throughput bioprocess design (HTBD). Biotechnol Bioeng 89(5):512–23. doi:10.1002/bit.20352
Rothschild LJ, Mancinelli RL (2001) Life in extreme environment. Nature 409:1092–1101
Rozzel JD (1999) Commercial scale biocatalysts: myths and reality. Bioorg Med Chem 7:2253–2261
Stroud A, Liddell S, Allers T (2012) Genetic and biochemical identification of a novel single-stranded DNA-binding complex in Haloferax volcanii. Front Microbiol 3:224. doi:10.3389/fmicb.2012.00224
Timpson LM, Alsafadi D, Mac Donnchadha C, Liddell S, Sharkey MA, Paradisi F (2012) Characterization of alcohol dehydrogenase (ADH12) from Haloarcula marismortui, an extreme halophile from the Dead Sea. Extremophiles 16(1):57–66. doi:10.1007/s00792-011-0405-0
Timpson LM, Liliensiek AK, Alsafadi D, Cassidy J, Sharkey MA, Liddell S, Allers T, Paradisi F (2013) A comparison of two novel alcohol dehydrogenase enzymes (ADH1 and ADH2) from the extreme halophile Haloferax volcanii. Appl Microbiol Biotechnol 97(1):195–203. doi:10.1007/s00253-012-4074-4
Tripepi M, You J, Temel S, Onder O, Brisson D, Pohlschroder M (2012) N-glycosylation of Haloferax volcanii flagellins requires known Agl proteins and is essential for biosynthesis of stable flagella. J Bacteriol 194(18):4876–87. doi:10.1128/JB.00731-12
Tripepi M, Esquivel RN, Wirth R, Pohlschroder M (2013) Haloferax volcanii cells lacking the flagellin FlgA2 are hypermotile. Microbiology 159(Pt 11):2249–58. doi:10.1099/mic.0.069617-0
van den Burg B (2003) Extremophiles as a source for novel enzymes. Curr Opin Microbiol 6(3):213–218. doi:10.1016/s1369-5274(03)00060-2
Wang Y, Cao H, Zhang G, Bougouffa S, Lee OO, Al-Suwailem A, Qian PY (2013) Autotrophic microbe metagenomes and metabolic pathways differentiate adjacent Red Sea brine pools. Sci Rep 3:1748. doi:10.1038/srep01748
Weuster-Botz D, Puskeiler R, Kusterer A, Kaufmann K, John GT, Arnold M (2005) Methods and milliliter scale devices for high-throughput bioprocess design. Bioprocess Biosyst Eng 28(2):109–19. doi:10.1007/s00449-005-0011-6
Wolfe AJ (2005) The acetate switch. Microbiol Mol Biol Rev 69(1):12–50. doi:10.1128/MMBR.69.1.12-50.2005
Acknowledgments
Research reported in this publication was supported by the King Abdullah University of Science and Technology (KAUST). The authors gratefully acknowledge the support of Eva Strillinger and Stefan Grötzinger by the International Graduate School of Science and Engineering (IGSSE), Technische Universität München (TUM), Germany. We thank Anastassja Akal, Ram Karan and Lars Janoscheck for their assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Eva Strillinger and Stefan Wolfgang Grötzinger contributed equally to this work.
Rights and permissions
About this article
Cite this article
Strillinger, E., Grötzinger, S.W., Allers, T. et al. Production of halophilic proteins using Haloferax volcanii H1895 in a stirred-tank bioreactor. Appl Microbiol Biotechnol 100, 1183–1195 (2016). https://doi.org/10.1007/s00253-015-7007-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7007-1