Abstract
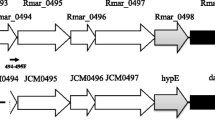
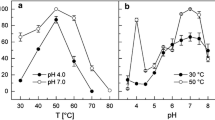
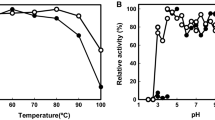
Two types of hetero-oligomeric dye-linked l-proline dehydrogenases (α4β4 and αβγδ types) are expressed in the hyperthermophilic archaea belonging to Thermococcales. In both enzymes, the β subunit (PDHβ) is responsible for catalyzing l-proline dehydrogenation. The genes encoding the two enzyme types form respective clusters that are completely conserved among Pyrococcus and Thermococcus strains. To compare the enzymatic properties of PDHβs from α4β4- and αβγδ-type enzyme complexes, eight PDHβs (four of each type) from Pyrococcus furiosus DSM3638, Pyrococcus horikoshii OT-3, Thermococcus kodakaraensis KOD1 JCM12380 and Thermococcus profundus DSM9503 were expressed in Escherichia coli cells and purified to homogeneity using one-step Ni-chelating chromatography. The α4β4-type PDHβs showed greater thermostability than most of the αβγδ-type PDHβs: the former retained more than 80 % of their activity after heating at 70 °C for 20 min, while the latter showed different thermostabilities under the same conditions. In addition, the α4β4-type PDHβs utilized ferricyanide as the most preferable electron acceptor, whereas αβγδ-type PDHβs preferred 2, 6-dichloroindophenol, with one exception. These results indicate that the differences in the enzymatic properties of the PDHβs likely reflect whether they were from an αβγδ- or α4β4-type complex, though the wider divergence observed within αβγδ-type PDHβs based on the phylogenetic analysis may also be responsible for their inconsistent enzymatic properties. By contrast, differences in the kinetic parameters among the PDHβs did not reflect the complex type. Interestingly, the k cat value for free α4β4-type PDHβ from P. horikoshii was much larger than the value for the same subunit within the α4β4-complex. This indicates that the isolated PDHβ could be a useful element for an electrochemical system for detection of l-proline.




Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Frew JE, Hill HA (1987) Electrochemical biosensors. Anal Chem 59:933A–944A
Kawakami R, Sakuraba H, Ohshima T (2004) Gene and primary structures of dye-linked l-proline dehydrogenase from the hyperthermophilic archaeon Thermococcus profundus show the presence of a novel heterotetrameric amino acid dehydrogenase complex. Extremophiles 8:99–108
Kawakami R, Sakuraba H, Tsuge H, Goda S, Katunuma N, Ohshima T (2005) A second novel dye-linked l-proline dehydrogenase complex is present in the hyperthermophilic archaeon Pyrococcus horikoshii OT-3. FEBS J 272:4044–4054
Kawakami R, Satomura T, Sakuraba H, Ohshima T (2012) l-Proline dehydrogenases in hyperthermophilic archaea: distribution, function, structure, and application. Appl Microbiol Biotechnol 93:83–93
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee YH, Nadaraia S, Gu D, Becker DF, Tanner JJ (2003) Structure of the proline dehydrogenase domain of the multifunctional PutA flavoprotein. Nat Struct Biol 10:109–114
Lee S, Jia B, Pham BP, Shao Y, Kwak JM, Cheong G-W (2012) Architecture and characterization of sarcosine oxidase from Thermococcus kodakaraensis KOD1. Extremophiles 16:87–93
Menzel R, Roth J (1981) Purification of the putA gene product. A bifunctional membrane-bound protein from Salmonella typhimurium responsible for the two-step oxidation of proline to glutamate. J Biol Chem 256:9755–9761
Monaghan PJ, Leys D, Scrutton NS (2007) Mechanistic aspects and redox properties of hyperthermophilic l-proline dehydrogenase from Pyrococcus furiosus related to dimethylglycine dehydrogenase/oxidase. FEBS J 274:2070–87
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Sakuraba H, Takamatsu Y, Satomura T, Kawakami R, Ohshima T (2001) Purification, characterization, and application of a novel dye-linked l-proline dehydrogenase from a hyperthermophilic archaeon, Thermococcus profundus. Appl Environ Microbiol 67:1470–1475
Sakuraba H, Satomura T, Kawakami R, Yamamoto S, Kawarabayasi Y, Kikuchi H, Ohshima T (2002) l-Aspartate oxidase is present in the anaerobic hyperthermophilic archaeon Pyrococcus horikoshii OT-3: characteristics and role in the de novo biosynthesis of nicotinamide adenine dinucleotide proposed by genomic sequencing. Extremophiles 6:275–281
Satomura T, Sakuraba H, Hara Y, Ohshima T (2010) Crystallization and preliminary X-ray analysis of a novel dye-linked l-proline dehydrogenase from the aerobic hyperthermophilic archaeon, Aeropyrum pernix. Acta Crytallogr Sect F Struct Biol Cryst Commun 66:1508–1510
Satomura T, Zhang X-D, Hara Y, Doi K, Sakuraba H, Ohshima T (2011) Characterization of a novel dye-linked l-proline dehydrogenase from an aerobic hyperthermophilic archaeon, Pyrobaculum calidifontis. Appl Microbiol Biotechnol 89:1075–1082
Scarpulla R, Soffer RL (1978) Membrane-bound proline dehydrogenase from Escherichia coli. Solubilization, purification, and characterization. J Biol Chem 253:5997–6001
Tanner JJ (2008) Structural biology of proline catabolism. Amino acids 35:719–730
Tsuge H, Kawakami R, Sakuraba H, Ago H, Miyano M, Aki K, Katunuma N, Ohshima T (2005) Crystal structure of a novel FAD-, FMN-, and ATP-containing l-proline dehydrogenase complex from Pyrococcus horikoshii. J Biol Chem 280:31045–31049
White TA, Krishnan N, Becker DF, Tanner JJ (2007) Structure and kinetics of monofunctional proline dehydrogenase from Thermus thermophilus. J Biol Chem 282:14316–14327
Zhang M, White TA, Schuermann JP, Baban BA, Becker DF, Tanner JJ (2004) Structures of the Escherichia coli PutA proline dehydrogenase domain in complex with competitive inhibitors. Biochemistry 43:12539–12548
Zheng H, Hirose Y, Kimura T, Suye S, Hori T, Katayama H, Arai J, Kawakami R, Ohshima T (2006) l-Proline sensor based on layer-by-layer immobilization of thermostable dye-linked l-proline dehydrogenase and polymerized mediator. Sci Technol Adv Mater 7:243–248
Zheng H, Lin L, Okezaki Y, Kawakami R, Sakuraba H, Ohshima T, Takagi K, Suye S (2010) Electrochemical behavior of dye-linked l-proline dehydrogenase on glassy carbon electrodes modified by multi-walled carbon nanotubes. Beilstein J Nanotechnol 1:135–141
Acknowledgments
We are grateful to Dr. Toshiaki Fukui (Tokyo Institute Technology) for providing the genome of T. kodakaraensis. This work was supported by KAKENHI (no. 21780099) to RK from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawakami, R., Noguchi, C., Higashi, M. et al. Comparative analysis of the catalytic components in the archaeal dye-linked l-proline dehydrogenase complexes. Appl Microbiol Biotechnol 97, 3419–3427 (2013). https://doi.org/10.1007/s00253-012-4201-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4201-2