Abstract
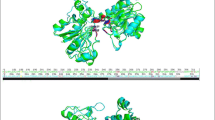
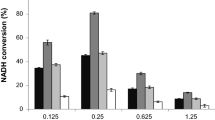
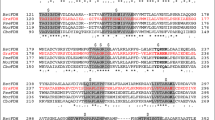
Formate dehydrogenases (FDHs) are frequently used for the regeneration of cofactors in biotransformations employing NAD(P)H-dependent oxidoreductases. Major drawbacks of most native FDHs are their strong preference for NAD+ and their low operational stability in the presence of reactive organic compounds such as α-haloketones. In this study, the FDH from Mycobacterium vaccae N10 (MycFDH) was engineered in order to obtain an enzyme that is not only capable of regenerating NADPH but also stable toward the α-haloketone ethyl 4-chloroacetoacetate (ECAA). To change the cofactor specificity, amino acids in the conserved NAD+ binding motif were mutated. Among these mutants, MycFDH A198G/D221Q had the highest catalytic efficiency (k cat/K m) with NADP+. The additional replacement of two cysteines (C145S/C255V) not only conferred a high resistance to ECAA but also enhanced the catalytic efficiency 6-fold. The resulting quadruple mutant MycFDH C145S/A198G/D221Q/C255V had a specific activity of 4.00 ± 0.13 U mg−1 and a K +m, NADP of 0.147 ± 0.020 mM at 30 °C, pH 7. The A198G replacement had a major impact on the kinetic constants of the enzyme. The corresponding triple mutant, MycFDH C145S/D221Q/C255V, showed the highest specific activity reported to date for a NADP+-accepting FDH (v max, 10.25 ± 1.63 U mg−1). However, the half-saturation constant for NADP+ (K +m, NADP , 0.92 ± 0.10 mM) was about one order of magnitude higher than the one of the quadruple mutant. Depending on the reaction setup, both novel MycFDH variants could be useful for the production of the chiral synthon ethyl (S)-4-chloro-3-hydroxybutyrate [(S)-ECHB] by asymmetric reduction of ECAA with NADPH-dependent ketoreductases.




Similar content being viewed by others
References
Andreadeli A, Platis D, Tishkov VI, Popov VO, Labrou NE (2008) Structure-guided alteration of coenzyme specificity of formate dehydrogenase by saturation mutagenesis to enable efficient utilization of NADP+. FEBS J 275:3859–3869
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Asano Y, Sekigawa T, Inukai M, Nakazawa A (1988) Purification and properties of formate dehydrogenase from Moraxella sp. strain C-1. J Bacteriol 170:3189–3193
Bisswanger (2008) Enzyme kinetics, 2nd edn. Wiley, Weinheim, p 120
Bräutigam S, Bringer-Meyer S, Weuster-Botz D (2007) Asymmetric whole cell biotransformations in biphasic ionic liquid/water-systems by use of recombinant Escherichia coli with intracellular cofactor regeneration. Tetrahedron-Asymmetry 18:1883–1887
Carugo O, Argos P (1997a) NADP-dependent enzymes. I: conserved stereochemistry of cofactor binding. Proteins 28:10–28
Carugo O, Argos P (1997b) NADP-dependent enzymes. II: evolution of the mono- and dinucleotide binding domains. Proteins 28:29–40
Eisenthal R, Danson MJ, Hough DW (2007) Catalytic efficiency and kcat/KM: a useful comparator? Trends Biotechnol 25:247–249
Fischer T, Pietruszka J (2010) Key building blocks via enzyme-mediated synthesis. Top Curr Chem 297:1–43
Galkin A, Kulakova L, Tishkov V, Esaki N, Soda K (1995) Cloning of formate dehydrogenase gene from a methanol-utilizing bacterium Mycobacterium vaccae N10. Appl Microbiol Biotechnol 44:479–483
Gul-Karaguler N, Sessions RB, Clarke AR, Holbrook J (2001) A single mutation in the NAD-specific formate dehydrogenase from Candida methylica allows the enzyme to use NADP. Biotechnol Lett 23:283–287
Hatrongjit R, Packdibamrung K (2010) A novel NADP+-dependent formate dehydrogenase from Burkholderia stabilis 15516: screening, purification and characterization. Enzyme Microb Tech 46:557–561
Hölsch K, Weuster-Botz D (2010a) New oxidoreductases from cyanobacteria: exploring nature’s diversity. Enzyme Microb Tech 47:228–235
Hölsch K, Weuster-Botz D (2010b) Enantioselective reduction of prochiral ketones by engineered bifunctional fusion proteins. Biotechnol Appl Biochem 56:131–140
Lamzin VS, Aleshin AE, Strokopytov BV, Yukhnevich MG, Popov VO (1992) Crystal structure of NAD-dependent formate dehydrogenase. Eur J Biochem 206:1011–1014
Lamzin VS, Dauter Z, Popov VO, Harutyunyan EH, Wilson KS (1994) High resolution structures of holo and apo formate dehydrogenase. J Mol Biol 236:759–785
Ma SK, Gruber J, Davis C, Newman L, Gray D, Wang A, Grate J, Huisman GW, Sheldon RA (2010) A green-by-design biocatalytic process for atorvastatin intermediate. Green Chem 12:81–86
Nanba H, Takaoka Y, Hasegawa J (2003) Purification and characterization of an α-haloketone-resistant formate dehydrogenase from Thiobacillus sp. strain KNK65MA, and cloning of the gene. Biosci Biotechnol Biochem 67:2145–2153
Olson BJ, Skavdahl M, Ramberg H, Osterman JC, Markwell J (2000) Formate dehydrogenase in Arabidopsis thaliana: characterization and possible targeting to the chloroplast. Plant Sci 159:205–212
Pereira R (1998) The use of baker’s yeast in the generation of asymmetric centers to produce chiral drugs and other compounds. Crit Rev Biotechnol 18:25–64
Popov VO, Lamzin VS (1994) NAD+-dependent formate dehydrogenase. Biochem J 301:625–643
Schütte H, Flossdorf J, Sahm H, Kula MR (1976) Purification and properties of formaldehyde dehydrogenase and formate dehydrogenase from Candida boidinii. Eur J Biochem 62:151–160
Serov AE, Popova AS, Fedorchuk VV, Tishkov VI (2002) Engineering of coenzyme specificity of formate dehydrogenase from Saccharomyces cerevisiae. Biochem J 367:841–847
Shimizu S, Kataoka M, Katoh M, Morikawa T, Miyoshi T, Yamada H (1990) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by a microbial aldehyde reductase in an organic solvent–water diphasic system. Appl Environ Microbiol 56:2374–2377
Slusarczyk H, Felber S, Kula MR, Pohl M (2000) Stabilization of NAD-dependent formate dehydrogenase from Candida boidinii by site-directed mutagenesis of cysteine residues. Eur J Biochem 267:1280–1289
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Tishkov VI, Popov VO (2004) Catalytic mechanism and application of formate dehydrogenase. Biochem Mosc 69:1252–1267
Tishkov VI, Popov VO (2006) Protein engineering of formate dehydrogenase. Biomol Eng 23:89–110
Tishkov VI, Galkin AG, Marchenko GN, Egorova OA, Sheluho DV, Kulakova LB, Dementieva LA, Egorov AM (1993) Catalytic properties and stability of a Pseudomonas sp. 101 formate dehydrogenase mutants containing Cys-255-Ser and Cys-255-Met replacements. Biochem Biophys Res Commun 192:976–981
van der Donk R, Zhao H (2003) Recent developments in pyridine nucleotide regeneration. Curr Opin Biotechnol 14:421–426
Weuster-Botz D (2007) Process intensification of whole-cell biocatalysis with ionic liquids. Chem Rec 7:334–340
Wichmann R, Vasic-Racki D (2005) Cofactor regeneration at the lab scale. Adv Biochem Engin/Biotechnol 92:225–260
Wierenga RK, de Maeyer MCH, Hol WGJ (1985) Interaction of pyrophosphate moieties with alpha-helixes in dinucleotide-binding proteins. Biochemistry 24:1346–1357
Wohlgemuth R (2010) Asymmetric biocatalysis with microbial enzymes and cells. Curr Opin Microbiol 13:283–292
Wu W, Zhu D, Hua L (2009) Site-saturation mutagenesis of formate dehydrogenase from Candida bodinii creating effective NADP+-dependent FDH enzymes. J Mol Catal B: Enzym 61:157–161
Yamamoto H, Matsuyama A, Kobayashi Y (2003) Synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate using fabG-homologues. Appl Microbiol Biotechnol 61:133–139
Yamamoto H, Mitsuhashi K, Kimoto N, Kobayashi Y, Esaki N (2005) Robust NADH-regenerator: improved alpha-haloketone-resistant formate dehydrogenase. Appl Microbiol Biotechnol 67:33–39
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 145 kb)
Rights and permissions
About this article
Cite this article
Hoelsch, K., Sührer, I., Heusel, M. et al. Engineering of formate dehydrogenase: synergistic effect of mutations affecting cofactor specificity and chemical stability. Appl Microbiol Biotechnol 97, 2473–2481 (2013). https://doi.org/10.1007/s00253-012-4142-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4142-9