Abstract
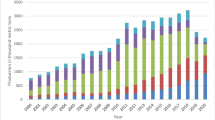
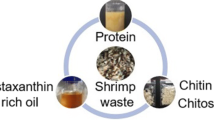
The production of shrimp waste from shrimp processing industries has undergone a dramatic increase in recent years. Continued production of this biomaterial without corresponding development of utilizing technology has resulted in waste collection, disposal, and pollution problems. Currently used chemical process releases toxic chemicals such as HCl, acetic acid, and NaOH into aquatic ecosystem as byproducts which will spoil the aquatic flora and fauna. Environmental protection regulations have become stricter. Now, there is a need to treat and utilize the waste in most efficient manner. The shrimp waste contains several bioactive compounds such as chitin, pigments, amino acids, and fatty acids. These bioactive compounds have a wide range of applications including medical, therapies, cosmetics, paper, pulp and textile industries, biotechnology, and food applications. This current review article present the utilization of shrimp waste as well as an alternative technology to replace hazardous chemical method that address the future trends in total utilization of shrimp waste for recovery of bioactive compounds.



Similar content being viewed by others
References
Aksu ZM (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026
Al-Azab AA (2005) Effect of partial and complete replacement of fish meal with locally produced shrimp meal on growth performance of Nile tilapia (Oreochromis niloticus). Egypt Nutr Feeds 8:1145–1156
Amar B, Philip R, Singh ISB (2000) Efficacy of fermented prawn shell waste as a feed ingredient for Indian prawn Fenneropenaeus indicus. Aquac Nutr 12:433–442
Anderson LK (1975) Extraction of carotenoid pigment from shrimp processing waste. US patent 3906112
Anonymous (1997) SEMARNAP. Anuario Estadistico de Pesca. Government of Mexico
Arcidiacono S, Kaplan DL (1992) Molecular weight distribution of chitosan isolated from Mucor rouxii under different culture and processing conditions. Biotechnol Bioeng 39:281–286
Armenta-López R, Guerrero I, Huerta S (2002) Astaxanthin extraction from shrimp waste by lactic fermentation and enzymatic hydrolysis of the carotenoprotein complex. J Food Sc 67:1002–1006
Bautista J, Jover M, Gutierrez JF, Corpas R, Cremadas O, Fontiveros E (2001) Preparation of crawfish chitin by in situ lactic acid production. Process Biochem 37:229–234
Bhaskar N, Suresh PV, Sakhare PZ, Sachindra NM (2007) Shrimp biowaste fermentation with Pediococcus acidolactici CFR2182: optimization of fermentation conditions by response surface methodology and effect of optimized conditions on deproteination/demineralization and carotenoids recovery. Enzym Microb Tech 40:1427–1434
Bhaskar N, Suresh PV, Sakhare PZ, Sachindra NM, Lalitha RG (2010) Yield and chemical composition of fractions from fermented shrimp biowaste. Waste Manage Res 28:64–70
Blanco M, Sotelo CG, Chapela MJ, Perez-Martin RI (2007) Towards sustainable and efficient use of fishery resources: present and future trends. Trends Food Sci Tech 18:29–36
Bodmeier R, Chen H, Paeratakul O (1989) A novel approach to the oral delivery of micro- or nanoparticles. Pharm Res 6:413–417
Bragagnolo L, Rodriguez-Amaya DB (2001) Total lipid cholesterol and fatty acids of farmed fresh water prawn (Macrobrachium rosenbergii) and wild marine shrimp (Penaeus brasiliensis, Penaeus schimitti, Xiphopenaeus kroyeri). J Food Compos Anal 14:359–369
Brandl F, Sommer F, Goepferich A (2007) Rational design of hydrogels for tissue engineering: impact of physical factors on cell behavior. Biomaterials 28:134–146
Britton G (1997) Proceedings of the 11th international symposium on carotenoids, Leiden, The Netherlands 1996. Pure Appl Chem 69:2027–2173
Calvo P, Remuñán-López C, Vila-Jato JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63:125–132
Cano-Lopez A, Simpson BK, Haard NF (1987) Extraction of carotenoprotein from shrimp process wastes with the aid of trypsin from Atlantic cod. J Food Sci 52:503–504
Carr FJ, Chill D, Maida N (2002) The lactic acid bacteria: a literature survey. Crit Rev Microbiol 28:281–370
Castillo R, Negre-sadargues G, Lenel R (1982) General survey of the carotenoids in crustacean. In: Britton G, Goodwin TW (eds) Carotenoid chemistry and biochemistry. Pergamon, Oxford, pp 211–224
Chen HM, Meyers SP (1982) Extraction of astaxanthin pigment from crawfish waste using a soy oil process. J Food Sci 47:892–896
Cheng JC, Pisano AP (2008) Photolithographic process for integration of the biopolymer chitosan into micro/nanostructures. J Microelectromech Syst 17:402–409
Cira LA, Huerta S, Hall GM, Shirai K (2002) Pilot scale lactic acid fermentation of shrimp wastes for chitin recovery. Process Biochem 37:1359–1366
Dai T, Tegos GP, Burkatovskaya M, Castano AP, Hamblin MR (2009) Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrob Agents Chemother 53:393–400
Dapkevicius MDLE, Batista I, Nout MJR, Rambouts FM, Houben JH (1998) Lipid and protein changes during ensilage of blue whiting (Micromesistius pooutassou risso) by acid and biological methods. Food Chem 63:147–152
Davies BH (1985) Carotenoid metabolism in animals: a biochemist's view. Pure Appl Chem 57:679–684
De Holanda HD, Netto FM (2006) Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. J Food Sci 71:298–303
De Jong AJ, Heidstra R, Spaink HP, Hartog MV, Meijer EA, Hendriks T, Schiavo FL, Terzi M, Bisseling T, Van Kammen A, De Vries SC (1993) Rhizobium lipooligosaccharides rescue a carrot somatic embryo mutant. Plant Cell 5:615–620
El Ghaouth A, Arul J, Asselin A, Benhamou N (1992) Antifungal activity of chitosan on post-harvest pathogens: induction of morphological and cytological alterations in Rhizopus stolonifer. Mycol Res 96:769–777
Erbacher P, Zou S, Bettinger T, Steffan AM, Remy JS (1998) Chitosan-based vector/DNA complexes for gene delivery: biophysical characteristics and transfection ability. Pharm Res 15:1332–1339
Evvyernie D, Yamazaki S, Morimoto K, Karita S, Kimura T, Sakka K, Ohmiya K (2000) Identification and characterization of Clostridium paraputrificum M-21, a chitinolytic, mesophilic and hydrogen-producing bacterium. J Biosci Bioeng 89:596–601
Fagberno OA (1996) Preparation properties and preservation of lactic acid fermented shrimp heads. Food Res Int 29:595–599
FAO/WHO (1985) Energy and protein requirements. Report of joint FAO/WHO/UNU expert Consultation Technical Report, vol 724. FAO/WHO and United Nations University, Geneva, pp 116–129
Feisal K, Montarop Y (2010) Chitin research revisited. Mar Drugs 8:1988–2012
Felix G, Baureithel K, Boller T (1998) Desensitization of the perception system for chitin fragments in tomato cells. Plant Physiol 117:643–650
Freier T, Montenegro R, Shan Koh H, Shoichet MS (2005) Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 26:4624–4632
Fuchs J, Martin JLM, An NT (1999) Impact of tropical shrimp aquaculture on the environment in Asia and the Pacific. Eur Comm Fish Bull 12:9–13
Gildberg A (2002) Enhancing returns from greater utilization. In: Bremner HA (ed) Safety and quality issues in fish processing. Woodhead publishing Ltd, Cambridge, pp 425–449
Gildberg A, Stenberg E (2001) A new process for advanced utilization of shrimp waste. Process Biochem 36:809–812
Gimeno M, Ramirez-Hernandez JY, Martinez-Ibarra C, Pacheco N, Garcia-Arrazola R, Barzana E, Shirai K (2007) One solvent extraction of astaxanthin from lactic acid fermented shrimp wastes. J Agric Food Chem 55:10345–10350
Gong Y, Gong L, Gu X, Ding F (2009) Chitooligosaccharides promote peripheral nerve regeneration in a rabbit common peronneal nerve crush injury model. Microsurgery 29:650–656
Gopalan NK, Dufresne A (2003) Crab shell chitin whisker reinforced natural rubber nanocomposites, processing and swelling behavior. Biomacromolecules 4:657–665
Guerard F, Sumaya-Martinez MT, Laroque D, Chabeaud A, Dufosse L (2007) Optimization of free radical scavenging activity by response surface methodology in the hydrolysis of shrimp processing discards. Process Biochem 42:1486–1491
Guerin M, Huntley Mark E, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends in Biochem 21:210–216
Guillou A, Khalila M, Adambounou I (1995) Effects of silage preservation on astaxanthin forms and fatty acid profiles of processed shrimp (Pandalus borealis) waste. Aquaculture 130:351–360
Haidar ZS, Hamdy RC, Tabrizian M (2008) Protein release kinetics for core-shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials 29:1207–1215
Hakim L, Sabarudin A, Oshita K, Oshima M, Motomizu S (2008a) Synthesis of cross-linked chitosan functionalized with threonine moiety and its application to on-line collection/concentration and determination of Mo, V and Cu. Talanta 74:977–985
Hakim L, Sabarudin A, Oshita K, Oshima M, Motomizu S (2008b) Synthesis of chitosan-based resins modified with tris (2-aminoethyl) amine moiety and its application to collection/concentration and determination of trace mercury by inductively coupled plasma atomic emission spectrometry. Talanta 76:1256–1260
Hall GM, DA Silva S (1992) Lactic acid fermentation of shrimp (Penaeus monodon) waste for chitin recovery. In: Brine CJ, Sandford PA, Zikakis JP (eds) Advances in chitin and chitosan. Elsevier Applied Sciences, London, pp 633–638
He H, Chen X, Sun C, Zhang Y, Gao P (2006) Preparation and functional evaluation of oligopeptide-enriched hydrolysate from shrimp (Acetes chinensis) treated with crude protease from Bacillus sp. SM98011. Biores Technol 97:85–90
Healy MG, Romo CR, Bustos R (1994) Bioconversion of marine crustacean shell waste. Res Conserv Recycl 11:139–147
Healy M, Green M, Healy A (2003) Bioprocessing of marine crustacean shell waste. Acta Biotechnol 23:151–160
Holland CR (1986) Recovery of single cell protein by chitosan in a batch dissolved air flocculation system. Dissertation, Department of mechanical and manufacturing, Aeronautical and chemical Engineering. The Queen's University of Belgast, Northern Ireland
Ito Y, Kaku H, Shibuya N (1997) Identification of a high-affinity binding protein for N-acetylchitooligosaccharide elicitor in the plasma membrane of suspension-cultured rice cells by affinity labeling. Plant J 12:347–356
Janes KA, Calvo P, Alonso MJ (2001) Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Deliv Rev 47:83–97
Je JY, Kim SK (2006) Antioxidant activity of novel chitin derivative. Bioorg Med Chem Lett 16:1884–1887
Jeuniaux C (1996) A brief survey of the early contribution of European scientists to chitin knowledge. In: Domard A, Jeuniaux C, Muzzarelli RAA, Roberts G (eds) Advances in chitin sciences. Jacques André Publication, Lyon, pp 1–9
Jollès P, Muzzarelli RAA (1999) Chitin and Chitinases, In: Birkhäuser Verlag (ed), Basel, Switzerland
Katayama T, Hirata K, Chichester CO (1971) The biosynthesis of astaxanthin IV. The carotenoids in the prawn Penaeus japonicas. Bull Jpn Soc Fish 37:614–620
Kelleher K (2005) Discards in the world's marine fisheries. An update FAO Fisheries Technical Paper N 470, Rome, pp131
Klokkevold PR, Fukayama H, Sung EC, Bertolami CN (1999) The effect of chitosan (poly-N-acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J Oral Maxillofac Surg 57:49–52
Knapczyk J, Brzozowski T (1982) Some biomedical properties of chitosan. Dissertation, Nicholas Copernicus Medical Academy, Krakow, Poland
Knorr D (1991) Recovery and utilization of chitin and chitosan in food processing waste management. Food Technol 26:114–122
Kyung-Taek OH, Young-Ju Kim, Van Nam N, Woo-Jin Jung, Ro-Dong Park (2007) Demineralization of crab shell waste by Pseudomonas aeruginosa F722. Process Biochem 42:1069–1074
Lang G, Clausen T (1985) The use of chitosan in cosmetics. Wella AG, Berlines allec 65, 6100, Darmstadt, West Germany
Latscha T (1990) Carotenoids—their nature and significance in animal feeds. In: F Hoffmann-La Roche Ltd, Animal Nutrition and Health, Basel, Switzerland. ISBN 3-906507-03-3. pp 110
Laura RC, Maria Del Carmen MC, Huerta S, Revah S, Shirai K (2006) Enzymatic hydrolysis of chitin in the production of oligosaccharide using Lecanicillium fungicola chitinases. Process Biochem 41:1106–1110
Legarrenta GI, Zakaria Z, Hall GM (1996) Lactic fermentation of prawn waste: comparison of commercial and isolated starter culture. In: Domard A, Jeuniaux C, Muzzarelli RAA, Roberts GAF (eds) Advances in chitin science, vol 1. Jacques Andre, Lyon, pp 399–406
Li H, Tyndale SJ, Heath DD, Letcher RJ (2005) Determination of carotenoids and all-trans-retinol on fish eggs by liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr 816:49–56
Liang TW, Chen YJ, Yen YH, Wang SL (2007) The antitumor activity of the hydrolysates of chitinous materials hydrolysed by crude enzyme from Bacillus amyloliquefaciens V656. Process Biochem 2:527–534
Luis SJT, Moldes AB, Alonso JL, Vazquez M (2003) Optimization of lactic acid production by Lactobacillus delbruckii through response surface methodology. J Food Sci 68:1454–1458
Maezaki Y, Tsuji K, Nakagawa Y, Kawai Y, Akimoto M, Tsugita T, Takekawa W, Terada A, Hara H, Mitsuoka T (1993) Hypocholesterolemic effect of chitosan in adult males. Biosci Biotech Biochem 57:1439–1444
Mahmoud NS, Ghaly AE, Arab F (2007) Unconventional approach for demineralization of deproteinized crustacean shells for chitin production. Am J Biochem Biotechnol 3:1–9
Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, Neves NM, Reis RL (2007) Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface 4:999–1030
Maryam Mizani A, Mahmood Aminlari B (2007) A new process for deproteinization of chitin from shrimp head waste. Proceedings of European Congress of Chemical Engineering (ECCE-6) 16–20
Mathew P, Nair KGR (2006) Ensilation of shrimp waste by Lactobacillus fermentum. Fish Technol 43:59–64
Mathur NK, Narang CK (1990) Chitin and chitosan versatile polysaccharides from marine animals. J Chem Educ 67:938
Meraz M, Shirai K, Larralde P, Revah S (1992) Studies on the bacterial acidification process of Cassava (Manihot esculenta). J Sci Food Agric 60:457–463
Mi FL, Shyu SS, Wu YB, Lee ST, Shyong JY, Huang RN (2001) Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 22:165–173
Millamena OM, Bautista MN, Kanazawa A (1996a) Methionine requirement of juvenile tiger shrimp Penaeus monodon Fabricius. Aquaculture 143:403–410
Millamena OM, Bautista MN, Kanazawa A (1996b) Valine requirement of post larval tiger shrimp Penaeus monodon Fabricius. Aquac Nutr 2:129–132
Millamena OM, Bautista MN, Kanazawa A (1997) Threonine requirement of juvenile tiger shrimp Penaeus monodon Fabricius. Aquaculture 151:9–14
Millamena OM, Bautista MN, Kanazawa A (1998) Requirement of juvenile tiger shrimp Penaeus monodon Fabricius for lysine and arginine. Aquaculture 164:95–104
Millamena OM, Bautista MN, Kanazawa A (1999) Quantitative dietary requirements of post larval tiger shrimp Penaeus monodon for histidine, isoleucine, leucine, phenylalanine and tryptophan. Aquaculture 179:169–179
Minami E, Kouchi H, Carlson RW, Cohn JR, Kolli VK, Day RB, Ogawa T, Stacey G (1996) Cooperative action of lipo-chitin nodulation signals on the induction of the early nodulin ENOD2 in soybean roots. Mol Plant Microbe Interact 9:574–583
Minami S, Suzuki H, Okamoto Y, Fujinaga T, Shigemasa Y (1998) Chitin and chitosan activate complement via the alternative pathway. Carbohyd Polym 36:151–155
Minke R, Blackwell J (1978) The structure of alpha-chitin. J Mol Biol 120:167–181
Maryam M, Aminlari M, Khodabandeh M (2005) An effective method for producing a nutritive protein extract powder from shrimp-head waste. Food Sci Technol Int 11:49–54
Morgan L, Chuenpagdue R (2003) Shifting gears addressing the collateral impacts of fishing methods in U.S. waters. Island Press, Washington
Morimoto K, Kimura T, Sakka K, Ohmiya K (2005) Over expression of a hydrogenase gene in Clostridium paraputrificum to enhance hydrogen gas production. FEMS Microbiol Lett 246:229–234
Mukundan MK, Antony PD, Nair MR (1986) A review on autolysis in fish. Fish Res 4:259–269
Muzzarelli RAA (1997) Chitin. Pergamon Press, New York, p 97
Muzzarelli RAA, Mozzarelli C (2009) Chitin and chitosan hydrogels. In: Phillips GO, Williams PA (eds) Handbook of Hydrocolloids. Wood head Publishing Ltd, Cambridge, pp 849–888
Naguib Yousry MA (2000) Antioxidant activities of astaxanthin and related carotenoids. J Agri Food Chem 48:1150–1154
Negre-Sadargues G, Castillo R, Petit H, Sance S, Martinez RG, Milicus JCG, Choubert G, Trilles JP (1993) Utilization of synthetic carotenoids by the prawn Penaeus japonicas reared under laboratory conditions. Aquaculture 110:151–159
Neith P, Garnica-Gonzalez M, Ramirez-Hernandez JY, Flores-Albino B, Gimeno M, Barzana E, Shirai K (2009) Effect of temperature on chitin and astaxanthin recoveries from shrimp waste using lactic acid bacteria. Biores Technol 100:2849–2854
NRC (1993) National Research Council—nutrient requirement of fish. National Academy of Science, Washington, p p124
Nwanna LC, Daramola (2001) Harnessing of shrimp head waste in Nigeria for low cost production of tilapia. Pak J Nutr 2:339–345
Okada M, Matsumura M, Shibuya N (2001) Identification of a high-affinity binding protein for N-acetylchitooligosaccharide elicitor in the plasma membrane from rice leaf and root cells. J Plant Physiol 158:121–124
Omum JV (1992) Shrimp waste—must it be wasted? Info fish Int 6:48
Ong SY, Wu J, Moochhala SM, Tan MH, Lu J (2008) Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 29:4323–4332
Oshita K, Seo K, Sabarudin A, Oshima M, Takayanagi T, Motomizu S (2008) Synthesis of chitosan resin possessing a phenylarsonic acid moiety for collection/concentration of uranium and its determination by ICP-AES. Anal Bioanal Chem 390:1927–1932
Ouattara B, Simard RE, Piette G, Begin A, Holley RA (2000) Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int J Food Microbiol 62:139–148
Park SH, KO YJ, Kim KS (2005) Physiological functions of chitosans as functional food ingredients. Korean J Chitin chitosan 10:55–60
Patat SA, Carnegie RB, Kingsbury C, Gross PS, Chapman R, Schey KL (2004) Antimicrobial activity of histones from hemocytes of the Pacific white shrimp. Eur J Biochem 271:4825–4833
Patidar P, Agarwal D, Banerjee T, Patil S (2005) Optimization of process parameters for chitinase production by soil isolates of Pencillium chrysogenum under solid substrate fermentation. Process Biochem 40:2962–2967
Peiselt da Silva KM, Pais da silva MI (2004) Copper sorption from diesel oil on chitin and chitosan polymers. Colloids Surf A 237:15–21
Percot AC, Viton Domard A (2003) Optimization of chitin extraction from shrimp shells. Biomacromolecules 4:12–18
Prameela K, Murali Mohan Ch, Hemalatha KPJ (2010a) Extraction of pharmaceutically important chitin and carotenoids from shrimp biowaste by microbial fermentation method. J Pharm Res 3:2393–2395
Prameela K, Murali Mohan Ch, Hemalatha KPJ (2010b) Optimization of fermentation of shrimp biowaste under different sources for recovery of chitin and carotenoids by using lactic acid bacteria. J Pharm Res 3:2888–2889
Prameela K, Murali Mohan CH, Smitha PV, Hemalatha KPJ (2010c) Bioremediation of shrimp biowaste by using natural probiotic for chitin and carotenoid production an alternative method to hazardous chemical method. Int J Appl Biol Pharm Technol 1:903–910
Prameela K, Murali Mohan CH, Hemalatha KPJ (2010d) Bio-efficiency of Pediococcus acidilactici (ATCC 8042) for recovery of chitin and carotenoids in the fermentation of shrimp biowaste. Int J ChemTech Res 2:1924–1928
Rao MS, Stevens WF (2005) Quality parameters of chitosan derived from fermentation of shrimp biomaterial using a drum reaction. J Chem Technol Biotechnol 80:1080–1087
Rao MS, Munoz J, Stevens WF (2000) Critical factors in chitin production by fermentation of shrimp biowastes. Appl Microbiol Biotechnol 54:808–813
Rao MS, Guyot JP, Pintado J, Stevens WF (2002) Advance in chitin Science. Vol. V, Bangkok, Thailand, 40–44
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Roberts GAF (1992) Chitin chemistry, Roberts, G.A; Macmillan press, Ltd, pp. 85–91
Rockway SW (2000) Absorbitol fat binding report. An in vitro binding study. Pharmanutrients, Scientific affairs pp. 1–8
Rosenberry B (1998) World shrimp farming no 11. Shrimp News International, SanDiego, p 328
Sachindra NM, Bhaskar N (2008) In vitro antioxidant activity of liquor from fermented shrimp biowaste. Biores Technol 99:9013–9016
Sachindra NM, Mahendrakar NS (2005) Process optimization for extraction of carotenoids from shrimp waste with vegetable oil. Biores Technol 96:1195–1200
Sachindra NM, Bhaskar N, Mahendrakar NS (2005) Carotenoids in different body components of Indian shrimps. J Sci Food Agric 85:167–172
Sachindra NM, Bhaskar N, Mahendrakar NS (2006) Recovery of carotenoids from shrimp waste in organic solvents. Waste Manag 26:1092–1098
Sachindra NM, Bhaskar N, Siddegoeda GS, Sathisha AD, Suresh PV (2007) Recovery of carotenoids from ensilaged shrimp waste. Biores Technol 98:1642–1646
Sakaguchi T, Horikoshi T, Nakajima A (1981) Adsorption of uranium by chitin phosphate and chitosan phosphate. Agric Biol Chem 45:2191–2195
Shahidi F, Synowiecki J (1991) Isolation and characterization of nutrients and value added products from snow crab (Chinoecetesopilio) and shrimp (Pandualus borealis) processing discards. J Agric Food Chem 39:1527–1532
Shahidi F, Metusalach, Brown JA (1998) Carotenoid pigments in seafood and aquaculture. CRC Cri Rev Food sci 38:1–67
Shirai K, Legarreta GI, Rodrigues-Serrano S, Huerta-Ochoa S, Saucedo Castaneda G, Hall GM (1997) Aspects of protein breakdown during the lactic acid fermentation of prawn waste. In: Domard A, Roberts GAF, Varum K (eds) Advances in chitin sciences, vol 2. Jacques Andre, lyon, pp 56–63
Shirai K, Guerrero I, Huerta S, Saucedo G, Castillo A, Gonzalez R (2001) Effect of initial glucose concentration and inoculation level of lactic acid bacteria in shrimp waste ensilation. Enz Microbiol Biotechnol 28:446–452
Sikorski P, Hori R, Wada M (2009) Revisit of alpha-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 10:1100–1105
Simpson BK, Haard NF (1985) The use of enzymes to extract carotenoprotein from shrimp waste. J Appl Biochem 7:212–222
Soltan MA, Hanafy MA, Wafa MIA (2008) An evaluation of fermented silage from fish by-products as a feed ingredient for African catfish (Claris gariepinus). Glob Vet 2:80–86
Srinivas Rao M, Willlem F, Stevens (2006) Fermentation of shrimp biowaste under different salt concentrations with amylolytic and non- amylolytic Lactobacillus strains for chitin production. Food Techno Biotechnol 44:83–87
Stanford PA (1987) Chitosan commercial uses and potential applications. Director Product Development Bio Applications Group, Protan, Inc, Woodinville
Stepnowski P, Olafsson G, Helgason H, Jasturff B (2004) Recovery of astaxanthin from seafood wastewater utilizing fish scales waste. Chemosphere 54:413–417
Struszczyk MH (2006) In: Monograph XI (ed) Global requirements for medical applications of chitin and its derivatives. Polish Chitin Society, Łódź, pp 95–102
Subasinghe S (1999) Chitin from shellfish waste-health benefits over-shadowing industrial uses. Info fish Int 3:58–65
Suzuki K, Mikami T, Okawa Y, Tokoro A, Suzuki S, Suzuki M (1986) Antitumor effects of hexa-N- acetylchitohexose and chitohexose. Carbohyd Res 151:403–408
Synowiecki J, Al-Khateeb NAAQ (2000) The recovery of protein hydrosylate during enzymatic isolation of chitin from shrimp Cragon cragon processing discards. J Food Chem 68:147–152
Synowiecki J, Al-Khateeb NA (2003) Production, properties and some new applications of chitin and its derivatives. Crit Rev Food Sci Nutr 43:145–171
Tacon AGJ, Akiyama DM (1997) Feed ingredients. In: D'Abramo JD, Conklin DE, Akiyama DM (eds) Crustacean Nutrition. Advances in World Aquaculture, vol 6. World Aquaculture Society, Baton Rouge, pp 411–472
Taha SMA, Swailam HMH (2002) Antibacterial activity of chitosan against Aeromonas hydrophila. Food/Nahrung 46:337–340
Tanaka T, Morishita Y, Suzui M, Kojima T, Okumura A, Mori H (1994) Chemopreservation of mouse urinary bladder carcinogenesis by naturally occurring carotenoid astaxanthin. Carcinogenesis 15:15–19
Vannuccini S (2004) Overview of fish production utilization consumption and trade: based on 2002 data. Food and Agriculture Organization of the United Nations, Rome
Wang SL, Chio SH (1998) Deproteinization of shrimp and crab shell with the protease of Pseudomonas aeruginosa K-187. Enzyme Microbiol Technol 22:629–633
Wang SL, Lin TY, Yen YH, Liao HF, Chen Y (2006) Bioconversion of shell fish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydrate Res 341:2507–2515
Wang SL, Lin HT, Liang TW, Chen YJ, Yen YH, Guo SP (2008) Reclamation of chitinous materials by bromelain for preparation of anti tumor and antifungal materials. Biores Technol 99:4386–4393
Wang B, Tian C, Wang L, Wang R, Fu H (2010) Chitosan: a green carbon source for the synthesis of graphitic nanocarbon, tungsten carbide and graphitic nanocarbon/tungsten carbide composites. Nanotechnology 21:025606
Wenhong C, Chaohua Z, Pengzhi H, Hongwu J, Jiming H, Jing Z (2009) Autolysis of shrimp head by gradual temperature and nutritional quality of the resulting hydrolysate. Food Sci Technol Leb 42:244–249
Woods B (1998) Microbiology of fermented foods, vol 1. Blackie, NY
Yamazaki S, Takegawa A, Kaneko Y, Kadokawa J, Yamagata M, Ishikawa M (2009) An acidic cellulose-chitin hybrid gel as novel electrolyte for an electric double layer capacitor. Electrochem Commun 11:68–70
Yang JK, Shih IL, Tzeng YM, Wang SL (2000) Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme Microbiol Technol 26:406–413
Zakaria Z, Hall GH, Shama G (1998) Lactic acid fermentation of scampi waste in a rotating horizontal bioreactor for chitin recovery. Process Biochem 33:1–6
Zia KM, Zuber M, Barikani M, Bhatti IA, Khan MB (2009) Surface characteristics of chitin-based shape memory polyurethane elastomers. Colloids Surf B Biointerfaces 72:248–252
Acknowledgements
KP is grateful to Prof. K. Aruna Lakshmi and Prof. R. Sinha for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kandra, P., Challa, M.M. & Kalangi Padma Jyothi, H. Efficient use of shrimp waste: present and future trends. Appl Microbiol Biotechnol 93, 17–29 (2012). https://doi.org/10.1007/s00253-011-3651-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3651-2