Abstract
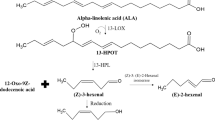
Green notes are substances that characterize the aroma of freshly cut grass, cucumbers, green apples, and foliage. In plants, they are synthesized by conversion of linolenic or linoleic acid via the enzymes lipoxygenase (LOX) and hydroperoxide lyase (HPL) to short-chained aldehydes. Current processes for production of natural green notes rely on plant homogenates as enzyme sources but are limited by low enzyme concentration and low specificity. In an alternative approach, soybean LOX2 and watermelon HPL were overexpressed in Saccharomyces cerevisiae. After optimization of the expression constructs, a yeast strain coexpressing LOX and HPL was applied in whole cell biotransformation experiments. Whereas addition of linolenic acid to growing cultures of this strain yielded no products, we were able to identify high green note concentrations when resting cells were used. The primary biotransformation product was 3(Z)-hexenal, a small amount of which isomerized to 2(E)-hexenal. Furthermore, both aldehydes were reduced to the corresponding green note alcohols by endogenous yeast alcohol dehydrogenase to some extent. As the cosolvent ethanol was the source of reducing equivalents for green note alcohol formation, the hexenal/hexenol ratio could be influenced by the use of alternative cosolvents. Further investigations to identify the underlying mechanism of the rather low biocatalyst stability revealed a high toxicity of linolenic acid to yeast cells. The whole cell catalyst containing LOX and HPL enzyme activity described here can be a promising approach towards a highly efficient microbial green note synthesis process.






Similar content being viewed by others
References
Akacha NB, Boubaker O, Gargouri M (2005) Production of hexenol in a two-enzyme system: kinetic study and modelling. Biotechnol Lett 27(23–24):1875–1878
Allmann S, Baldwin IT (2010) Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 329(5995):1075–1078
Belitz H-D, Grosch W, Schieberle P (2009) Food Chemistry, vol 4th ed. Springer
Bourel G, Nicaud J-M, Nthangeni B, Santiago-Gomez P, Belin J-M, Husson F (2004) Fatty acid hydroperoxide lyase of green bell pepper: cloning in Yarrowia lipolytica and biogenesis of volatile aldehydes. Enzyme Microb Technol 35(4):293–299
Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14(2):115–132
Brunerie P, Koziet Y (1997) Process for producing natural cis-3-hexenol from unsaturated fatty acids. US Patent 5620879
Chernoff YO, Vincent A, Liebman SW (1994) Mutations in eukaryotic 18S ribosomal RNA affect translational fidelity and resistance to aminoglycoside antibiotics. EMBO J 13(4):906–913
Cipak A, Hasslacher M, Tehlivets O, Collinson EJ, Zivkovic M, Matijevic T, Wonisch W, Waeg G, Dawes IW, Zarkovic N, Kohlwein SD (2006) Saccharomyces cerevisiae strain expressing a plant fatty acid desaturase produces polyunsaturated fatty acids and is susceptible to oxidative stress induced by lipid peroxidation. Free Radic Biol Med 40(5):897–906
Do TQ, Schultz JR, Clarke CF (1996) Enhanced sensitivity of ubiquinone-deficient mutants of Saccharomyces cerevisiae to products of autoxidized polyunsaturated fatty acids. Proc Natl Acad Sci USA 93(15):7534–7539
Entian K-D, Kötter P (1998) Yeast mutant and plasmid collections. In: Brown AJP, Tuite MF (eds) Yeast Gene Analysis, vol 26. Methods in Microbiology. Academic Press Ltd., San Diego, pp 431–449
Erhart E, Hollenberg CP (1983) The presence of a defective LEU2 gene on 2 mu DNA recombinant plasmids of Saccharomyces cerevisiae is responsible for curing and high copy number. J Bacteriol 156(2):625–635
Fauconnier ML, Marlier M (1996) An efficient procedure for the production of fatty acid hydroperoxides from hydrolyzed flax seed oil and soybean lipoxygenase. Biotechnol Tech 10(11):839–844. doi:10.1007/bf00154668
Fauconnier ML, Mpambara A, Delcarte J, Jacques P, Thonart P, Marlier M (1999) Conversion of green note aldehydes into alcohols by yeast alcohol dehydrogenase. Biotechnol Lett 21(7):629–633
Fukushige H, Hildebrand DF (2005a) A simple and efficient system for green note compound biogenesis by use of certain lipoxygenase and hydroperoxide lyase sources. J Agric Food Chem 53(17):6877–6882
Fukushige H, Hildebrand DF (2005b) Watermelon (Citrullus lanatus) hydroperoxide lyase greatly increases C6 aldehyde formation in transgenic leaves. J Agric Food Chem 53(6):2046–2051
Gardini F, Lanciotti R, Guerzoni ME (2001) Effect of trans-2-hexenal on the growth of Aspergillus flavus in relation to its concentration, temperature and water activity. Lett Appl Microbiol 33(1):50–55
Gardner HW (1991) Recent investigations into the lipoxygenase pathway of plants. Biochim Biophys Acta 1084(3):221–239
Gargouri M, Akacha NB, Legoy MD (2004) Coupled hydroperoxide lyase and alcohol dehydrogenase for selective synthesis of aldehyde or alcohol. Appl Biochem Biotechnol 119(2):171–180
Götz-Schmidt EM, Wenzel M, Schreier P (1986) C-6-volatiles in homogenates from green leaves. Localization of hydroperoxide lyase activity. Lebensm-Wiss Technol 19:152–155
Green DW, Sun HW, Plapp BV (1993) Inversion of the substrate specificity of yeast alcohol dehydrogenase. J Biol Chem 268(11):7792–7798
Hauml UA, Silke N, Lerch K, Muheim A (2001) Hydroperoxide lyase. US Patent 6238898
Hornung E, Walther M, Kuhn H, Feussner I (1999) Conversion of cucumber linoleate 13-lipoxygenase to a 9-lipoxygenating species by site-directed mutagenesis. Proc Natl Acad Sci USA 96(7):4192–4197
Imai T, Ohno T (1995) The relationship between viability and intracellular pH in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol 61(10):3604–3608
Knust B, von Wettstein D (1992) Expression and secretion of pea-seed lipoxygenase isoenzymes in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 37(3):342–351
Larroy C, Fernandez MR, Gonzalez E, Pares X, Biosca JA (2002a) Characterization of the Saccharomyces cerevisiae YMR318C (ADH6) gene product as a broad specificity NADPH-dependent alcohol dehydrogenase: relevance in aldehyde reduction. Biochem J 361(Pt 1):163–172
Larroy C, Pares X, Biosca JA (2002b) Characterization of a Saccharomyces cerevisiae NADP(H)-dependent alcohol dehydrogenase (ADHVII), a member of the cinnamyl alcohol dehydrogenase family. Eur J Biochem 269(22):5738–5745
Lopes TS, Klootwijk J, Veenstra AE, van der Aar PC, van Heerikhuizen H, Raué HA, Planta RJ (1989) High-copy-number integration into the ribosomal DNA of Saccharomyces cerevisiae: a new vector for high-level expression. Gene 79(2):199–206
Mosblech A, Feussner I, Heilmann I (2009) Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem 47(6):511–517
Muller B, Gautier A, Dean C, Kuhn J-C (1995) Process for the enzymatic preparation of aliphatic alcohols and aldehydes from linoleic acid, linolenic acid, or a natural precursor
Navarro-Avino JP, Prasad R, Miralles VJ, Benito RM, Serrano R (1999) A proposal for nomenclature of aldehyde dehydrogenases in Saccharomyces cerevisiae and characterization of the stress-inducible ALD2 and ALD3 genes. Yeast 15(10A):829–842
Noordermeer MA, Veldink GA, Vliegenthart JFG (1999) Alfalfa contains substantial 9-hydroperoxide lyase activity and a 3Z:2E-enal isomerase. FEBS Lett 443(2):201–204
Noordermeer MA, Van Der Goot W, Van Kooij AJ, Veldsink JW, Veldink GA, Vliegenthart JF (2002) Development of a biocatalytic process for the production of c6-aldehydes from vegetable oils by soybean lipoxygenase and recombinant hydroperoxide lyase. J Agric Food Chem 50(15):4270–4274
Rabetafika HN, Gigot C, Fauconnier ML, Ongena M, Destain J, du Jardin P, Wathelet JP, Thonart P (2008) Sugar beet leaves as new source of hydroperoxide lyase in a bioprocess producing green-note aldehydes. Biotechnol Lett 30(6):1115–1119
Sambrook J, Russell DW (2001) Molecular cloning - a laboratory manual. Molecular cloning - a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Santiago-Gómez MP, Vergely C, Policar C, Nicaud J-M, Belin J-M, Rochette L, Husson F (2007) Characterization of purified green bell pepper hydroperoxide lyase expressed by Yarrowia lipolytica: Radicals detection during catalysis. Enzyme Microb Technol 41(1–2):13–18
Schade F, Thompson JE, Legge RL (2003) Use of a plant-derived enzyme template for the production of the green-note volatile hexanal. Biotechnol Bioeng 84(3):265–273
Schiestl RH, Gietz RD (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet 16(5–6):339–346
Sekiya J, Kajiwara T, Munechika K, Hatanaka A (1983) Distribution of lipoxygenase and hydroperoxide lyase in the leaves of various plant species. Phytochemistry 22(9):1867–1869
Shirano Y, Shibata D (1990) Low temperature cultivation of Escherichia coli carrying a rice lipoxygenase L-2 cDNA produces a soluble and active enzyme at a high level. FEBS Lett 271(1–2):128–130
Sund H, Theorell H (1963) In: Boyer PD (ed) The Enzymes, 2nd edn. Academic Press, New York, pp 57–83
Takamura H, Gardner HW (1996) Oxygenation of (3Z)-alkenal to (2E)-4-hydroxy-2-alkenal in soybean seed (Glycine max L.). Biochim Biophys Acta 1303(2):83–91
Valli M, Sauer M, Branduardi P, Borth N, Porro D, Mattanovich D (2005) Intracellular pH distribution in Saccharomyces cerevisiae cell populations, analyzed by flow cytometry. Appl Environ Microbiol 71(3):1515–1521
Vörös K, Feussner I, Kuhn H, Lee J, Graner A, Lobler M, Parthier B, Wasternack C (1998) Characterization of a methyljasmonate-inducible lipoxygenase from barley (Hordeum vulgare cv. Salome) leaves. Eur J Biochem 251(1–2):36–44
Whitehead IM, Slusarenko AJ, Waspi U, Gaskin DJH, Brash AR, Tijet N (2001) Guava (Psidium guajava) 13-hydroperoxide lyase and uses thereof. W O Patent 99/58648
Yesilirmak F, Sayers Z (2009) Heterelogous expression of plant genes. Int J Plant Genomics 2009:296482
Zahedi RP, Sickmann A, Boehm AM, Winkler C, Zufall N, Schonfisch B, Guiard B, Pfanner N, Meisinger C (2006) Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol Biol Cell 17(3):1436–1450
Acknowledgments
The authors acknowledge Wilfried Schwab for providing plasmids containing LOX and HPL cDNAs. Financial support from Arbeitsgemeinschaft Industrieller Fördervereinigungen e.V. under the project 15088N is gratefully acknowledged.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buchhaupt, M., Guder, J.C., Etschmann, M.M.W. et al. Synthesis of green note aroma compounds by biotransformation of fatty acids using yeast cells coexpressing lipoxygenase and hydroperoxide lyase. Appl Microbiol Biotechnol 93, 159–168 (2012). https://doi.org/10.1007/s00253-011-3482-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3482-1