Abstract
Cone snails are marine predators that use venoms to immobilize their prey. The venoms of these mollusks contain a cocktail of peptides that mainly target different voltage- and ligand-gated ion channels. Typically, conopeptides consist of ten to 30 amino acids but conopeptides with more than 60 amino acids have also been described. Due to their extraordinary pharmacological properties, conopeptides gained increasing interest in recent years. There are several conopeptides used in clinical trials and one peptide has received approval for the treatment of pain. Accordingly, there is an increasing need for the production of these peptides. So far, most individual conopeptides are synthesized using solid phase peptide synthesis. Here, we describe that at least some of these peptides can be obtained using prokaryotic or eukaryotic expression systems. This opens the possibility for biotechnological production of also larger amounts of long chain conopeptides for the use of these peptides in research and medical applications.
Similar content being viewed by others
Introduction
Cone snails are mainly known due to the beauty of their shells, which can be found in plenty of the “sea side souvenir shops” all over the world. Commonly not so well recognized is that the biology of these marine snails is very fascinating because these slow animals live as predators. All of the about 500 species known do hunt animals, e.g., other snails, worms, or even fish (see Fig. 1). The immobilization of the prey results from the action of relative complex venoms, which are injected into the victims by using harpoon like teeth. The venom of each species contains up to 200 pharmacologically active components that mainly target different voltage- and ligand-gated ion channels (for overviews see: Olivera et al. 1990; Olivera 2002; Terlau and Olivera 2004). With respect to the venom action on the victim, the different conopeptides can be grouped according to their biological role for the immobilization of prey (for more details see: Olivera 1997). Some conopeptides have been shown to be important for the fast immobilization of the prey (“lightning strike cabal”) whereas others exert their action during later phases of the envenomation, which results in an irreversible block of neuromuscular transmission (“motor cabal”). Because of their special pharmacological properties conopeptides have gained increasing interest in recent years. Moreover, since several conopeptides are currently being tested within clinical trials and the ω-conotoxin MVIIA has obtained an approval as an analgesic drug (Ziconitide, Prialt®) there is an increasing need to produce conopeptides in larger quantities.
Conus purpurascens hunting a clown fish. The snail stings the fish with a harpoon like tooth, which is hollow and barbed and held at the tip of the proboscis. Upon venom injection (upper right) the fish is immobilized within less than a few seconds (lower left) and engulfed by the snail (lower right; originally from Terlau et al. 1996)
In this review, we briefly describe the properties of conopeptides and discuss the current and potential applications of conopeptides in research and clinics. Furthermore, we describe ways of biotechnological production of these peptides, which will help to ease the use of conopeptides within different fields of research and other potential applications.
Properties of conopeptides
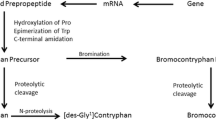
Conopeptides are genetically encoded small proteins that are initially translated as prepropeptide precursors consisting of a signal sequence (the “pre” region) followed by a “pro” region, and at the C-terminal part representing the mature toxin region. The pharmacological active peptide is generated by an enzymatic cleavage of the C-terminal part of the prepropeptide. With respect to their amino acid sequence, conopeptides can be classified in two broad classes: the disulfide-rich peptides and the non-disulfide-rich peptides (Terlau and Olivera 2004).
For the disulfide-rich peptides, several superfamilies have been defined according to the difference in their cysteine connectivity. Within these superfamilies, different peptides may have different targets. Therefore, a given superfamily may consist of several conotoxin families grouped according to their pharmacological properties. The targets of conopeptides are usually voltage-gated and ligand-gated ion channels. For example, within the O-superfamily of disulfide-rich conopeptides that contain six cysteines with an arrangement also known from peptides of different origins (the “inhibitor cystine knot” motif), at least four different families have been identified: the δ-conotoxins, known to inhibit the fast inactivation of voltage-gated Na+ channels, the μO-conotoxins that inhibit voltage-gated Na+ currents, the κ-conotoxins which interact with voltage-gated K+ channels, and the ω-conotoxins known to block voltage-activated Ca++ channels. In terms of the immobilization of prey, the peptides from these different families can be involved in the lightning strike cabal or in the motor cabal. For the fish hunting species, Conus purpurascens, for example, it has been shown that the δ-conotoxins PVIA and the κ-conotoxin PVIIA are crucial for the lightning strike cabal (Terlau et al. 1996).
One of the most striking features of conopeptides is their pharmacological properties: conopeptides are known to be extraordinarily potent and highly specific. The ω-conotoxin MVIIA, for example, specifically targets N-Type Ca++ channels (Cav2.2) with little affinity to other Ca++ channel subtypes (Olivera et al. 1987; Hillyard et al. 1992). Since N-type Ca++ channels are primarily located in the presynaptic space, the action of ω-conotoxin MVIIA results in blocking synaptic transmission and therefore during envenomation of prey, this peptide is involved in the motor cabal. With respect to the action of the whole venom, the extraordinary specificity of the conopeptides indicates that every single peptide is a “specialist” optimized for a certain target and that only the concerted action of the different peptides present in the venom results in the biological action needed for the achievement of the predatory life of these snails. Due to these pharmacological properties, conopeptides can be ideal tools to study the structure and function of a given target (see below). The finding that peptides with similar cysteine connectivity target different families of ion channels demonstrates that even minor changes in the overall structure of these peptides can result in major pharmacological differences. In fact, these properties might help for the understanding of pharmacological specificity for ion-channel subtypes (i.e., K+ channel vs. Na+ channel).
Investigations on the venom composition of cone snails suggest that during evolution, every single species of cone snails has developed its own set of conopeptides and it has been estimated that probably more than 50,000 different conopeptides can be found in the venoms of all cone snail species (Terlau and Olivera 2004). Accordingly, conopeptides are extremely variable in their amino acid sequences and the pharmacological properties of only a few of these conopeptides have been characterized in detail so far. The current idea on how the snails have evolved these highly specific peptides within their 50 million years of evolution is that for the cysteine-rich peptides, a hypermutation has occurred for the amino acids in between these cysteines of the mature toxin. Interestingly, in contrast to the mature toxin, the signal sequence of the precursor peptides within a superfamily gene is highly conserved (Olivera 1997, 2006). This high degree of homology can be used for the identification of new peptides from the same family by using PCR techniques.
For the biological function of the cysteine-rich peptides, their disulfide bridges are known to be essential. Furthermore, the disulfide bridges represent a backbone for the structure of these peptides, which are usually relatively stable. The structure of several conopeptides has been solved either by NMR or X-ray analysis.
A striking feature of conopeptides is the presence of a variety of posttranslational modifications, which include hydroxylation of prolines, carboxylation of glutamate, d-amino acids, or sulfated tyrosine (Buczek et al. 2005). There are indications that cone snails express the enzymes important for those modifications in their venom ducts (Stanley et al. 1997; Bandyopadhyay et al. 1998). For γ-carboxylation, it was shown that the enzyme has a recognition signal in the pro region of the precursor. The functional importance of these posttranslational modifications is only partially understood but for the biotechnological production of conopeptides, these modifications introduce some limitations (see below).
Applications of conopeptides
The extraordinary pharmacological specificity of conopeptides resulted in an intensive use of these peptides for different applications. The ω-conotoxins, for example, are heavily used in neuroscience and also in other areas of research to study the function of Ca++-channel subtypes. Accordingly, for the most widely used ω-conotoxin, GVIA, there are about 2,000 research papers published using this peptide as a tool to study these channels.
Due to their specific binding properties, conopeptides can also be valuable tools to obtain structural information about their corresponding target. Especially for transmembrane proteins where no or little structural information is available, the interaction of peptidic toxins can be used to gain structural information of the target protein by using the interaction surface of the peptide as a fingerprint of the interaction surface of the target. Besides conopeptides, also specific toxins from other organisms like scorpions or snakes are used for these studies which have paved the way for obtaining the first structural data on K+ channels (Miller 1995; Doyle et al. 1998; MacKinnon et al. 1998). Interestingly, most recent data obtained by solid-state NMR suggest that obviously during the binding of peptide toxins to their target, some conformational changes on both binding partners occur (Lange et al. 2006). These results indicate that at least some conformational dynamics take place during drug–target interactions.
Since voltage-gated and ligand-gated ion channels as the target proteins of conopeptides are involved in a variety of different physiological functions, it becomes immediately clear that certain conopeptides do have the potential to act as lead compounds for new drugs. Accordingly, several conopeptides are currently undergoing clinical trials. Since the application of peptides as drugs is limited it is clear that not always the actual peptide will be the substance of choice for a given indication. But nevertheless, these peptides will help our understanding in how substances do interact with their target and maybe, thereby, opening new fields of pharmaceutical research.
The first example of a conopeptide that is used as a drug is ω-conotoxin MVIIA (Ziconitide, Prialt®) from Conus magus, which has been approved for the treatment of intractable pain (Miljanich 2004; Staats et al. 2004; Stix 2005). Interestingly, ω-conotoxin MVIIA is one of the first substances from a marine organism that became a drug used in clinics.
Several other conopeptides are currently being explored as antinociceptive agents. The analgesic effect can be mediated through the interaction with different targets including voltage-activated Ca++ channels and Na+ channels but also nicotinic acetylcholine receptors, neurotensin receptors, NMDA-type glutamate receptors, and norepinephrine transporter (Olivera 2006). This demonstrates that the specific action of conopeptides can interfere within different signaling pathways involved in pain sensation. In addition, the analgetic action of these peptides helps in the understanding of the molecular mechanisms involved in pain. Only through the discovery that ω-conotoxins are analgetic, for example, did it become clear that N-Type Ca++ channels are a potential drug target for severe pain.
Besides their use as pain killers, conopeptides might also be useful for other clinical indications. For κ-conotoxin PVIIA (see Fig. 2), for example, it has been demonstrated that it reduces the size of the myocardial infarct in an ischemia/reperfusion model in rabbits, rats, and dogs in vivo (Zhang et al. 2003; Lubbers et al. 2005). An important feature of this cardioprotective effect is that the reduction was not only observed when κ-PVIIA was applied prior to ischemia, but also if given after the ischemic event and prior to reperfusion, which reflects more the clinical situation.
Sequence and structure of K+ channel binding conotoxins. Despite a different cysteine backbone and different structures all the three peptides κ-PVIIA (pdb code: 1AV3); κM-RIIIK (*) and Conk-S1 (pdb code: 2CA7) interact with Shaker K+ channels by occluding the ion channel pore (Shon et al. 1998; Jacobsen et al. 2000; Ferber et al. 2003; Al-Sabi et al. 2004; Bayrhuber et al. 2005; Verdier et al. 2005). O 4-hydroxyproline; Z pyroglutamate; # denotes an amidated C-terminal amino acid. * Structural coordinates obtained from T. Carlomagno
These examples demonstrate that the biomedical potential of conopeptides is established and that it is very likely that due to the current research on the characterization of their properties, further conopeptides with very interesting pharmacological properties will be discovered.
Biotechnological production of conopeptides
From the natural source, conotoxins can only be obtained in tiny quantities that limit their availability for research and medical applications. To obtain larger amounts of these peptides, two basic approaches are available: chemical synthesis and recombinant production in heterologous expression systems (Fig. 3). Due to the posttranslational modifications of many conotoxins described above (Craig et al. 1999; Buczek et al. 2005), chemical synthesis via solid phase peptide synthesis (SPPS) on a resin support (Merrifield 1963) has been the method of choice to produce conotoxins in large quantities. SPPS using the orthogonal 9-Fluorenylmethyloxycarbonyl (Fmoc)/tertiary-Butyl (tBu) chemistry (Chang and Meienhofer 1978) allows the use of piperidine and trifluoroacetic acid to remove the N-terminal Fmoc and the side chain protection groups, respectively. These low-hazard reagents allow the synthesis of peptides also in typical biology lab environments, making SPPS peptide synthesis available to many life scientists. After removal of the protection groups and cleavage from the resin, the linear peptide needs to be refolded to generate the correct secondary and tertiary structure including the formation of the correct disulfide bond pattern between the cysteine residues present in the polypeptide chain. This oxidation step is usually performed with molecular oxygen (Rudolph and Lilie 1996). The yield of correctly formed disulfide bonds in this step is usually low but can be considerably increased by performing thiol-disulfide exchange reactions with low-molecular-weight thiols that are added in reduced and oxidized form. Typical “oxido-shuffling” reagents are the combinations of reduced and oxidized glutathione, cysteine and cystine, cysteamine and cystamine or di-β-hydroxyethyl disulfide, and 2-mercaptoethanol (Rudolph and Lilie 1996; Bulaj 2005; Lovelace et al. 2006). The thiol–disulfide exchange takes place by nucleophilic attack of the thiolate anion. Therefore, the folding reaction of the disulfide-rich conotoxin peptides is usually performed at mildly alkaline conditions (Moroder et al. 1996; Rudolph and Lilie 1996). SPPS is well functioning for the synthesis up to 30-mer peptides. As most known conotoxins consist of about 10–30 amino acid residues (Terlau and Olivera 2004), SPPS is perfectly suitable for their production. Classical examples for conotoxins synthesized by SPPS are the Ca++-channel inhibitor ω-Conotoxin GVIA (Rivier et al. 1987) and µ-Conotoxin GIIIA (Becker et al. 1989), an inhibitor of voltage-dependent Na+ channels. Many other conotoxins with posttranslationally modified amino acids have been successfully produced by SPPS in the last 20years.
Ways to biotechnological production of disulfide-rich peptides. Solid phase peptide synthesis of disulfide-rich peptides requires subsequent folding of the peptide fragment in the presence of oxygen. Addition of low-molecular-weight thiols in oxidized and reduced form (oxido-shuffling reagents) to the folding solution can help to increase the yield of correctly folded peptide. Recombinant peptide production can result in insoluble protein (inclusion bodies) or soluble protein. From inclusion bodies folding in the presence of oxido-shuffling reagents can be successful
Chemical ligation of purified peptide fragments can be used to produce longer polypeptides containing up to several hundred amino acids (Dawson and Kent 2000). Using this technique, Conkunitzin-S1 (Conk-S1; see Fig. 2) was recently synthesized (Dy et al. 2006). This conopeptide belongs to a new class of conotoxins that do not contain posttranslational modifications apart from C-terminal amidation and that are structurally strongly related to Kunitz proteins (Bayrhuber et al. 2005; Dy et al. 2006). The expenses for the chemicals and protected amino acids to synthesize such large peptides are quite high. A cost-effective alternative approach to produce such peptides in larger quantities is the use of bacterial and eukaryotic expression systems. The limited posttranslational machinery of these expression systems does not permit the production of peptides with the kind of posttranslational modifications found in many smaller conopeptides (Craig et al. 1999; Buczek et al. 2005). Escherichia coli is the best characterized and most widely used bacterial host for the production of recombinant proteins (Baneyx 1999; Pines and Inouye 1999). The reasons are the low costs for culturing E. coli and the short culturing times. In general, small peptides are difficult to overexpress directly in E. coli since they are either quickly degraded by cellular proteases or they accumulate to form insoluble aggregates, so-called inclusion bodies (Georgiou and Valax 1996). In addition, the cytoplasm of E. coli is a reducing environment that prevents the formation of disulfide bonds (Prinz et al. 1997), further increasing the tendency to form inclusion bodies upon overexpression. To achieve at least high expression levels of these small peptides, expressing them in fusion with larger, well-expressing carrier proteins has been the most successful approach so far (Butt et al. 1989; Malakhov et al. 2004). The use of highly soluble carrier proteins like, e.g., maltose-binding protein (Kapust and Waugh 1999), thioredoxin (Lavallie et al. 1993), or glutathione S-transferase (Nygren et al. 1994) can even lead to the expression of soluble fusion proteins. Through affinity chromatography targeting such carrier proteins, these soluble fusion proteins can often be easily purified (Nygren et al. 1994; Esposito and Chatterjee 2006; Peti and Page 2007). The additional use of protease-deficient host strains (Maurizi 1992; Gottesman 1996) can help to avoid non-specific proteolytic degradation. From the refolded or soluble fusion protein, the peptide has to be released by chemical or proteolytic cleavage at a pre-engineered cleavage site located in the protein sequence between the carrier protein and the target peptide sequence (Esposito and Chatterjee 2006). Following this approach, the medically important ω-conotoxin MVIIA (Ziconitide) was produced in soluble form as a fusion protein with glutathione S-transferase (Xia et al. 2006). Very recently, the newly discovered Conotoxin lt7a was produced in soluble form in fusion with thioredoxin (Pi et al. 2007).
When soluble expression can not be achieved, the protein must be solubilized from the inclusion bodies with denaturing reagents like urea and guanidinium hydrochloride and subsequently refolded by dilution into or by dialysis against a target buffer containing no denaturant (Fahnert et al. 2004). Similar to the formation of disulfide bonds in chemically synthesized peptides (see above), the yield of this refolding step can often be strongly increased by addition of oxido-shuffling reagents (Rudolph and Lilie 1996). In this way, the recombinant conopeptide Conk-S1 has been refolded (Bayrhuber et al. 2006; see also below).
Alternatively, strains with knocked-off oxidoreductases like, e.g., E. coli Origami (Novagen) offer a moderately oxidizing environment in their cytoplasm and can be used to increase the fraction of correctly folded disulfide-bonded proteins (Prinz et al. 1997). Although it seems that expression levels tend to be low in such strains (Xiong et al. 2005), this approach has been successfully used for soluble expression of proteins with disulfide bonds (for example, see Lauber et al. 2001; Lehmann et al. 2003; Rabhi-Essafi et al. 2007). But a successful expression of small disulfide-rich peptides like the conotoxins has not been reported so far in such a strain.
Another possibility to obtain soluble fusion proteins of disulfide-rich peptides is the expression into the oxidative environment of the periplasm using a signal peptide sequence in the expression construct. This approach has been successfully used for the periplasmic expression of dendrotoxins and scorpion toxins as fusion proteins with maltose-binding protein (Ducancel et al. 1989; Legros et al. 1997; Legros et al. 2001). More recently, peptides with the inhibitor cystine knot (ICK) structural motif have even been successfully secreted into the culture medium by expressing them as barnase fusion proteins (Schmoldt et al. 2005). Periplasmic expression approaches have not been successfully used for the small disulfide-rich conotoxins. Oxidative folding in the periplasmic space seems to work efficiently only for specific disulfide bridge patterns.
In most fusion protein expression vectors with engineered protease cleavage site, the cloning sites for the peptide coding sequence are located downstream of the coding sequence for the protease recognition site. Therefore, the proteolytic release of peptides from their fusion partner has the potential disadvantage that the cleaved-off peptide may contain additional N-terminal amino acid residues due to cloning. Proteases may also cleave at sites different from the engineered cleavage site and destroy the peptide of interest. In addition, the expenses for proteases can account for a considerable portion of the production costs of recombinant peptides. Chemical cleavage, e.g., by CNBr at methionine residues placed between the fusion partner and the peptide sequence, apart from requiring high lab-safety standards, can also happen at other methionine residues that may be in the peptide.
The production of peptides as fusion proteins with inteins is a way to avoid potential problems with proteolytic or chemical cleavage. Inteins are segments of proteins that are able to excise themselves by a self-catalytic mechanism and rejoin the two flanking parts, the N- and C-exteins, with a peptide bond (Perler and Adam 2000; Saleh and Perler 2006). This splicing reaction is typically initiated by an N to S or N to O acyl shift of Cys1 or Ser1 at the N-terminus of the intein. The resulting (thio)ester is attacked by the first residue of the C-extein, leading via several intermediates to the formation of a new peptide bond between the exteins. For biotechnological purposes, modified inteins have been designed. For example, in the pTWIN vectors (New England Biolabs), the N-terminal cysteine of intein Ssp/ DnaB (Wu et al. 1998) has been changed to alanine to block the splicing reaction. This mutant intein is still able to undergo temperature and pH dependent cleavage of the peptide bond between the C-terminus of the intein and the downstream amino acid, thus releasing the C-terminally fused target protein (Mathys et al. 1999; Wood et al. 1999). The design of the multiple cloning site downstream of the Ssp/ DnaB coding sequence in the pTWIN vectors includes a SapI restriction site that allows the attachment of the peptide sequence immediately downstream of the intein splice site, thus not leaving any additional N-terminal residues in the peptide after the splice reaction. With such an N-terminal intein fusion construct, recombinant Conk-S1 has been produced (Bayrhuber et al. 2006). The Ssp/ DnaB–Conk-S1 fusion protein was expressed in insoluble form and was refolded in a buffer containing oxidized and reduced glutathione as oxido-shuffling reagents (Bayrhuber et al. 2006). Cleavage of the fusion protein was achieved by shift to lower pH (from 7.5 to 6.5) and incubation overnight at room temperature. This type of expression construct was also successfully used for the production of another recombinant conkunitzin (unpublished results) and of recombinant Kaliotoxin (Lange et al. 2006), a potassium channel inhibitor from the venom of the scorpion Leiurus quinquestriatus.
In summary, the production of disulfide-bonded proteins in E. coli frequently includes the refolding of these proteins from inclusion bodies or secretion into the periplasmic space. Both approaches tend to decrease the final yield. An alternative to the production in E. coli is the production of disulfide-bonded proteins in eukaryotic expression systems. The methylotrophic yeast, Pichia pastoris, provides an intracellular folding environment similar to mammalian cells and secretion into the surrounding medium can be easily accomplished (White et al. 1994). Using this expression system, a number of highly disulfide-bonded proteins have been produced (Kristensen et al. 1999; Cabral et al. 2003). The level of secretion per cell is fairly low in P. pastoris (White et al. 1994). Therefore, high-density fermentation was originally a requirement to produce larger amounts of secreted proteins. More recently, the coexpression of protein disulfide isomerase, which probably reduces the retention of disulfide-bonded proteins in the endoplasmic reticulum, has led to enormous improvements of expression yields also in less dense cultures (Vad et al. 2005; Tsai et al. 2006; Huo et al. 2007). Similar to E. coli, P. pastoris requires no special equipment for handling and the growth media are not more expensive than those for E. coli. Thus, production of disulfide-bonded proteins in P. pastoris has developed into an attractive alternative to production in E. coli. But a successful production of conopeptides has not been reported so far. Insect cells are also used for the production of disulfide-bonded proteins with the Baculovirus system (Vogel et al. 2004; Galesi et al. 2007). Stably transfected Drosophila melanogaster S2 cells (Galesi et al. 2007) are another insect cell expression system. Recently, psalmotoxin 1, an acid-sensing ion channel inhibitor, was successfully produced in a stable D. melanogaster S2 cell line (Escoubas et al. 2003). Due to the high costs of insect cell media, a more wide-spread use of insect cells for the production of highly disulfide-bonded peptides is currently unlikely.
A central consideration of biotechnological protein production is cost-effectiveness. Of all approaches described here, production in E. coli is the least expensive because of the very low costs for the culture media and the low investments necessary for culturing these bacteria. Thus, despite of the difficulties encountered, especially with the production of highly disulfide-bonded proteins and peptides like, e.g., the conopeptides, E. coli remains the system of choice for their recombinant production. Only the yeast, P. pastoris, having the advantage compared to E. coli of providing an intracellular folding environment similar to that of mammalian cells, provides an excellent alternative to bacterial expression systems. Other eukaryotic expression systems like, e.g., insect cells or even mammalian cells require prohibitively expensive culture media and expensive cell culture equipment. Regarding the large group of small conotoxins with posttranslational modifications, their production is, at present, still mostly confined to chemical synthesis, e.g., by SPPS. In vitro systems comprising posttranslational modification enzymes to transform recombinant precursors into bioactive peptides have been successfully used (Ozawa et al. 2007). But at present, they offer no higher cost-effectiveness compared to the chemical synthesis and may only be used in the future in cases where refolding of chemically synthesized, highly disulfide-bonded peptides with modified amino acids has not been successful.
Altogether, for the majority of the presently known conopeptides, chemical synthesis is the most important method to produce large amounts of peptide. Recombinant production remains confined to conopeptides without posttranslational modifications. The rapid development of these methods in recent years may allow the biosynthetic production of some modified peptides in the future.
References
Al-Sabi A, Lennartz D, Ferber M, Gulyas J, Rivier JE, Olivera BM, Carlomagno T, Terlau H (2004) KappaM-conotoxin RIIIK, structural and functional novelty in a K channel antagonist. Biochemistry 43:8625–8635
Bandyopadhyay PK, Colledge CJ, Walker CS, Zhou LM, Hillyard DR, Olivera BM (1998) Conantokin-G precursor and its role in gamma-carboxylation by a vitamin K-dependent carboxylase from a Conus snail (vol 273, pg 5447, 1998). J Biol Chem 273:14658–14658
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Current Opinion in Biotechnology 10:411–421
Bayrhuber M, Vijayan V, Ferber M, Graf R, Korukottu J, Imperial J, Garrett JE, Olivera BM, Terlau H, Zweckstetter M et al (2005) Conkunitzin-S1 is the first member of a new Kunitz-type neurotoxin family. Structural and functional characterization. J Biol Chem 280:23766–23770
Bayrhuber M, Graf R, Ferber M, Zweckstetter M, Imperial J, Garrett JE, Olivera BM, Terlau H, Becker S (2006) Production of recombinant Conkunitzin-S1 in Escherichia coli. Protein Expr Purif 47:640–644
Becker S, Atherton E, Gordon RD (1989) Synthesis and characterization of mu-conotoxin IIIa. Eur J Biochem 185:79–84
Buczek O, Bulaj G, Olivera BM (2005) Conotoxins and the posttranslational modification of secreted gene products. Cell Mol Life Sci 62:3067–3079
Bulaj G (2005) Formation of disulfide bonds in proteins and peptides. Biotechnol Adv 23:87–92
Butt TR, Jonnalagadda S, Monia BP, Sternberg EJ, Marsh JA, Stadel JM, Ecker DJ, Crooke ST (1989) Ubiquitin fusion augments the yield of cloned gene-products in Escherichia coli. Proc Natl Acad Sci USA 86:2540–2544
Cabral KMS, Almeida MS, Valente AP, Almeida FCL, Kurtenbach E (2003) Production of the active antifungal Pisum sativum defensin 1 (Psd1) in Pichia pastoris: overcoming the inefficiency of the STE13 protease. Protein Expr Purif 31:115–122
Chang CD, Meienhofer J (1978) Solid-phase peptide-synthesis using mild base cleavage of nalpha-fluorenylmethyloxycarbonylamino acids, exemplified by a synthesis of dihydrosomatostatin. Int J Pept Protein Res 11:246–249
Craig AG, Bandyopadhyay P, Olivera BM (1999) Post-translationally modified neuropeptides from Conus venoms. Eur J Biochem 264:271–275
Dawson PE, Kent SBH (2000) Synthesis of native proteins by chemical ligation. Ann Rev Biochem 69:923–960
Doyle DA, Cabral JM, Pfuetzner RA, Kuo AL, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998) The structure of the potassium channel: molecular basis of K conduction and selectivity. Science 280:69–77
Ducancel F, Boulain JC, Tremeau O, Menez A (1989) Direct expression in E. coli of a functionally active protein A-snake toxin fusion protein. Protein Eng 3:139–143
Dy CY, Buczek P, Imperial JS, Bulaj G, Horvath MP (2006) Structure of conkunitzin-S1, a neurotoxin and Kunitz-fold disulfide variant from cone snail. Acta Crystallogr, D Biol Crystallogr 62:980–990
Escoubas P, Bernard C, Lambeau G, Lazdunski M, Darbon H (2003) Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci 12:1332–1343
Esposito D, Chatterjee DK (2006) Enhancement of soluble protein expression through the use of fusion tags. Curr Opin Biotechnol 17:353–358
Fahnert B, Lilie H, Neubauer P (2004) Inclusion bodies: formation and utilisation. Adv Biochem Eng Biotechnol 89:93–142
Ferber M, Sporning A, Jeserich G, DelaCruz R, Watkins M, Olivera BM, Terlau H (2003) A novel Conus peptide ligand for K channels. J Biol Chem 278(4):2177–2183
Galesi AL, Pereira CA, Moraes AM (2007) Culture of transgenic Drosophila melanogaster Schneider 2 cells in serum-free media based on TC100 basal medium. Biotechnol J 2:1399–1407
Georgiou G, Valax P (1996) Expression of correctly folded proteins in Escherichia coli. Curr Opin Biotechnol 7:190–197
Gottesman S (1996) Proteases and their targets in Escherichia coli. Annu Rev Genet 30:465–506
Hillyard DR, Monje VD, Mintz IM, Bean BP, Nadasdi L, Ramachandran J, Miljanich G, Azimizoonooz A, Mcintosh JM, Cruz LJ et al (1992) A new Conus peptide ligand for mammalian presynaptic Ca2 channels. Neuron 9:69–77
Huo XD, Liu YY, Wang X, Ouyang PK, Niu ZD, Shi YH, Qiu BS (2007) Co-expression of human protein disulfide isomerase (hPDI) enhances secretion of bovine follicle-stimulating hormone (bFSH) in Pichia pastoris. Protein Expr Purif 54:234–239
Jacobsen RB, Koch ED, Lange-Malecki B, Stocker M, Verhey J, Van Wagoner RM, Vyazovkina A, Olivera BM, Terlau H (2000) Single amino acid substitutions in kappa-conotoxin PVIIA disrupt interaction with the Shaker K channel. J Biol Chem 275:24639–24644
Kapust RB, Waugh DS (1999) Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci 8:1668–1674
Kristensen AK, Brunstedt J, Nielsen JE, Mikkelsen JD, Roepstorff P, Nielsen KK (1999) Processing, disulfide pattern, and biological activity of a sugar beet defensin, AX2, expressed in Pichia pastoris. Protein Expr Purif 16:377–387
Lange A, Giller K, Hornig S, Martin-Eauclaire MF, Pongs O, Becker S, Baldus M (2006) Toxin-induced conformational changes in a potassium channel revealed by solid-state NMR. Nature 440:959–962
Lauber T, Marx UC, Schulz A, Kreutzmann P, Rosch P, Hoffmann S (2001) Accurate disulfide formation in Escherichia coli: overexpression and characterization of the first domain (HF6478) of the multiple Kazal-type inhibitor LEKTI. Protein Expr Purif 22:108–112
Lavallie ER, Diblasio EA, Kovacic S, Grant KL, Schendel PF, Mccoy JM (1993) A thioredoxin gene fusion expression system that circumvents inclusion body formation in the Escherichia coli cytoplasm. Bio-Technology 11:187–193
Legros C, Feyfant E, Sampieri F, Rochat H, Bougis PE, Martin-Eauclaire MF (1997) Influence of a NH2-terminal extension on the activity of KTX2, a K channel blocker purified from Androctonus australis scorpion venom. FEBS Lett 417:123–129
Legros C, Kaabi H, El Ayeb M, Ceard B, Vacher H, Bougis PE, Martin-Eauclaire MF (2001) Use of fusion protein constructs to generate potent immunotherapy and protection against scorpion toxins. Vaccine 20:934–942
Lehmann K, Hoffmann S, Neudecker P, Suhr M, Becker WM, Rosch P (2003) High-yield expression in Escherichia coli, purification, and characterization of properly folded major peanut allergen Ara h 2. Protein Expr Purif 31:250–259
Lovelace ES, Armishaw CJ, Colgrave ML, Wahlstrom ME, Alewood PF, Daly NL, Craik DJ (2006) Cyclic MrIA: a stable and potent cyclic conotoxin with a novel topological fold that targets the norepinephrine transporter. J Med Chem 49:6561–6568
Lubbers NL, Campbell TJ, Polakowski JS, Bulaj G, Layer RT, Moore J, Gross GJ, Cox BF (2005) Postischemic administration of CGX-1051, a peptide from cone snail venom, reduces infarct size in both rat and dog models of myocardial ischemia and reperfusion. J Cardiovasc Pharmacol 46:141–146
MacKinnon R, Cohen SL, Kuo AL, Lee A, Chait BT (1998) Structural conservation in prokaryotic and eukaryotic potassium channels. Science 280:106–109
Malakhov MP, Mattern MR, Malakhova OA, Drinker M, Weeks SD, Butt TR (2004) SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics 5:75–86
Mathys S, Evans TC, Chute IC, Wu H, Chong S, Benner J, Liu XQ, Xu MQ (1999) Characterization of a self-splicing mini-intein and its conversion into autocatalytic N- and C-terminal cleavage elements: facile production of protein building blocks for protein ligation. Gene 231:1–13
Maurizi MR (1992) Proteases and protein-degradation in Escherichia coli. Experientia 48:178–201
Merrifield RB (1963) Solid phase peptide synthesis. 1. Synthesis of a tetrapeptide. J Am Chem Soc 85:2149–2154
Miljanich GP (2004) Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem 11:3029–3040
Miller C (1995) The charybdotoxin family of K channel-blocking peptides. Neuron 15:5–10
Moroder L, Besse D, Musiol HJ, RudolphBohner S, Siedler F (1996) Oxidative folding of cystine-rich peptides vs regioselective cysteine pairing strategies. Biopolymers 40:207–234
Nygren PA, Stahl S, Uhlen M (1994) Engineering proteins to facilitate bioprocessing. Trends Biotechnol 12:184–188
Olivera BM (1997) EE Just lecture, 1996—Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Mol Biol Cell 8:2101–2109
Olivera BM (2002) Conus venom peptides: reflections from the biology of clades and species. Ann Rev Ecol Syst 33:25–47
Olivera BM (2006) Conus peptides: biodiversity-based discovery and exogenomics. J Biol Chem 281:31173–31177
Olivera BM, Cruz LJ, Desantos V, Lecheminant GW, Griffin D, Zeikus R, Mcintosh JM, Galyean R, Varga J, Gray WR et al (1987) Neuronal calcium-channel antagonists—discrimination between calcium-channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry 26:2086–2090
Olivera BM, Rivier J, Clark C, Ramilo CA, Corpuz GP, Abogadie FC, Mena EE, Woodward SR, Hillyard DR, Cruz LJ (1990) Diversity of Conus neuropeptides. Science 249:257–263
Ozawa A, Cai Y, Lindberg I (2007) Production of bioactive peptides in an in vitro system. Anal Biochem 366:182–189
Perler FB, Adam E (2000) Protein splicing and its applications. Curr Opin Biotechnol 11:377–383
Peti W, Page R (2007) Strategies to maximize heterologous protein expression in Escherichia coli with minimal cost. Protein Expr Purif 51:1–10
Pi C, Liu J, Wang L, Jiang X, Liu Y, Peng C, Chen S, Xu A (2007) Soluble expression, purification and functional identification of a disulfide-rich conotoxin derived from Conus litteratus. J Biotechnol 128:184–193
Pines O, Inouye M (1999) Expression and secretion of proteins in E. coli. Mol Biotechnol 12:25–34
Prinz WA, Aslund F, Holmgren A, Beckwith J (1997) The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J Biol Chem 272:15661–15667
Rabhi-Essafi I, Sadok A, Khalaf N, Fathallah DM (2007) A strategy for high-level expression of soluble and functional human interferon alpha as a GST-fusion protein in E. coli. Protein Eng Des Sel 20:201–209
Rivier J, Galyean R, Gray WR, Azimi-Zonooz A, McIntosh JM, Cruz LJ, Olivera BM (1987) Neuronal calcium channel inhibitors. Synthesis of omega-conotoxin GVIA and effects on 45Ca uptake by synaptosomes. J Biol Chem 262:1194–1198
Rudolph R, Lilie H (1996) In vitro folding of inclusion body proteins. Faseb J 10:49–56
Saleh L, Perler FB (2006) Protein splicing in cis and in trans. Chem Rec 6:183–193
Schmoldt HU, Wentzel A, Becker S, Kolmar H (2005) A fusion protein system for the recombinant production of short disulfide bond rich cystine knot peptides using barnase as a purification handle. Protein Expr Purif 39:82–89
Shon KJ, Stocker M, Terlau H, Stuhmer W, Jacobsen R, Walker C, Grilley M, Watkins M, Hillyard DR, Gray WR et al (1998) kappa-Conotoxin PVIIA is a peptide inhibiting the shaker K channel. J Biol Chem 273:33–38
Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, Fisher R, Bryce DA, Mangieri EA, Luther RR et al (2004) Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. Jama 291:63–70
Stanley TB, Stafford DW, Olivera BM, Bandyopadhyay PK (1997) Identification of a vitamin K-dependent carboxylase in the venom duct of a Conus snail. Febs Lett 407:85–88
Stix G (2005) A toxin against pain. Sci Am 292:88–93
Terlau H, Olivera BM (2004) Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol Rev 84:41–68
Terlau H, Shon KJ, Grilley M, Stocker M, Stuhmer W, Olivera BM (1996) Strategy for rapid immobilization of prey by a fish-hunting marine snail. Nature 381:148–151
Tsai CW, Duggan PF, Shimp RL, Miller LH, Narum DL (2006) Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. J Biotechnol 121:458–470
Vad R, Nafstad E, Dahl LA, Gabrielsen OS (2005) Engineering of a Pichia pastoris expression system for secretion of high amounts of intact human parathyroid hormone. J Biotechnol 116:251–260
Verdier L, Al-Sabi A, Rivier JEF, Olivera BM, Terlau H, Carlomagno T (2005) Identification of a novel pharmacophore for peptide toxins interacting with K channels. J Biol Chem 280:21246–21255
Vogel CW, Fritzinger DC, Hew BE, Thorne M, Bammert H (2004) Recombinant cobra venom factor. Mol Immunol 41:191–199
White CE, Kempi NM, Komives EA (1994) Expression of highly disulfide-bonded proteins in Pichia pastoris. Structure 2:1003–1005
Wood DW, Wu W, Belfort G, Derbyshire V, Belfort M (1999) A genetic system yields self-cleaving inteins for bioseparations. Nat Biotechnol 17:889–892
Wu H, Xu MQ, Liu XQ (1998) Protein trans-splicing and functional mini-inteins of a cyanobacterial dnaB intein. Biochim Biophys Acta 1387:422–432
Xia Z, Chen Y, Zhu Y, Wang F, Xu X, Zhan J (2006) Recombinant omega-conotoxin MVIIA possesses strong analgesic activity. BioDrugs 20:275–281
Xiong S, Wang YF, Ren XR, Li B, Zhang MY, Luo Y, Zhang L, Xie QL, Su KY (2005) Solubility of disulfide-bonded proteins in the cytoplasm of Escherichia coli and its “oxidizing” mutant. World J Gastroenterol 11:1077–1082
Zhang SJ, Yang XM, Liu GS, Cohen MV, Pemberton K, Downey JM (2003) CGX-1051, a peptide from conus snail venom, attenuates infarction in rabbit hearts when administered at reperfusion. J Cardiovasc Pharmacol 42:764–771
Acknowledgement
The authors are grateful to Harald Kolmar for his comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Becker, S., Terlau, H. Toxins from cone snails: properties, applications and biotechnological production. Appl Microbiol Biotechnol 79, 1–9 (2008). https://doi.org/10.1007/s00253-008-1385-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1385-6