Abstract
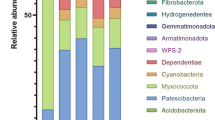
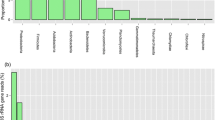
Bacterial communities are important catalysts in the production of composts. Here, it was analysed whether the diversity of bacteria in finished composts is stable and specific for the production process. Single-strand conformation polymorphism (SSCP) based on polymerase chain reaction amplified partial 16S rRNA genes was used to profile and analyse bacterial communities found in total DNA extracted from finished composts. Different batches of compost samples stored over a period of 12 years and a 1-year-old vermicompost were compared to each other. According to digital image analysis, clear differences could be detected between the profiles from compost and vermicompost. Differences between three different periods of compost storage and between replicate vermicompost windrows were only minor. A total of 41 different 16S rRNA genes were identified from the SSCP profiles by DNA sequencing, with the vast majority related to yet-uncultivated bacteria. Sequences retrieved from compost mainly belonged to the phyla Actinobacteria and Firmicutes. In contrast, vermicompost was dominated by bacteria related to uncultured Chloroflexi, Acidobacteria, Bacteroidetes and Gemmatimonadetes. The differences were underscored with specific gene probes and Southern blot hybridizations. The results confirmed that different substrates and composting processes selected for specific bacterial communities in the finished products. The specificity and consistency of the bacterial communities inhabiting the compost materials suggest that cultivation-independent bacterial community analysis is a potentially useful indicator to characterize the quality of finished composts in regard to production processes and effects of storage conditions.



Similar content being viewed by others
References
Albiach R, Canet R, Pomares F, Ingelmo F (2001) Organic matter components and aggregate stability after the application of different amendments to a horticultural soil. Bioresour Technol 76:125–129
Alfreider A, Peters S, Tebbe CC, Rangger A, Insam H (2002) Microbial community dynamics during composting of organic matter as determined by 16S ribosomal DNA analysis. Compost Sci Util 10:303–312
Anastasi A, Varese GC, Voyron S, Scannerini S, Marchisio VF (2004) Characterization of fungal biodiversity in compost and vermicompost. Compost Sci Util 12:185–191
Bailey KL, Lazarovits G (2003) Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res 72:169–180
Barker AV (1997) Composition and uses of compost. ACS Sym Ser 668:140–162
Bolta SV, Mihelic R, Lobnik F, Lestan D (2003) Microbial community structure during composting with and without mass inocula. Compost Sci Util 11:6–15
Brosius J, Dull TJ, Sleeter DD, Noller HF (1981) Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol 148:107–127
Buckley DH, Schmidt TM (2003) Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environ Microbiol 5:441–452
Cahyani VR, Watanabe A, Matsuya K, Asakawa S, Kimura M (2002) Succession of microbiota estimated by phospholipid fatty acid analysis and changes in organic constituents during the composting process of rice straw. Soil Sci Plant Nutr 48:735–743
Dees PM, Ghiorse WC (2001) Microbial diversity in hot synthetic compost as revealed by PCR-amplified rRNA sequences from cultivated isolates and extracted DNA. FEMS Microbiol Ecol 35:207–216
Dohrmann AB, Tebbe CC (2004) Microbial community analysis by PCR-single-strand conformation polymorphism (PCR-SSCP). In: Kowalchuk GA, de Bruijn FJ, Head IM, Akkermans AD, van Elsas JD (eds) Molecular microbial ecology manual, 2nd edn. Kluwer, Dordrecht, pp 809–838
Dominguez J, Edwards CA, Subler S (1997) A comparison of vermicomposting and composting. Biocycle 38:57–59
Edwards CA (1995) Historical overview of vermicomposting. Biocycle 36:56–58
Farrelly V, Rainey FA, Stackebrandt E (1995) Effect of genome size and rrn gene copy number on PCR amplification of 16S ribosomal RNA genes from a mixture of bacterial species. Appl Environ Microbiol 61:2798–2801
Furlong MA, Singleton DR, Coleman DC, Whitman WB (2002) Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl Environ Microbiol 68:1265–1279
Gupta RS (2004) The phylogeny and signature sequences characteristics of Fibrobacteres, Chlorobi, and Bacteroidetes. Crit Rev Microbiol 30:123–143
Hassen A, Belguith K, Jedidi N, Cherif A, Cherif M, Boudabous A (2001) Microbial characterization during composting of municipal solid waste. Bioresour Technol 80:217–225
Janssen PH, Yates PS, Grinton BE, Taylor PM, Sait M (2002) Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl Environ Microbiol 68:2391–2396
Joseph SJ, Hugenholtz P, Sangwan P, Osborne CA, Janssen PH (2003) Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl Environ Microbiol 69:7210–7215
Juteau P, Tremblay D, Villemur R, Bisaillon JG, Beaudet R (2004) Analysis of the bacterial community inhabiting an aerobic thermophilic sequencing batch reactor (AT-SBR) treating swine waste. Appl Microbiol Biotechnol 66:115–122
Kowalchuk GA, Os GJ, Aartrijk J, Veen JA (2003) Microbial community responses to disease management soil treatments used in flower bulb cultivation. Biol Fert Soils 37:55–63
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Marshall MN, Cocolin L, Mills DA, VanderGheynst JS (2003) Evaluation of PCR primers for denaturing gradient gel electrophoresis analysis of fungal communities in compost. J Appl Microbiol 95:934–948
McCarthy AJ, Williams ST (1992) Actinomycetes as agents of biodegradation in the environment—a review. Gene 115:189–192
Noble R, Coventry E (2005) Suppression of soil-borne plant diseases with composts: a review. Biocontrol Sci Technol 15:3–20
Peters S, Koschinsky S, Schwieger F, Tebbe CC (2000) Succession of microbial communities during hot composting as detected by PCR-single-strand-conformation polymorphism-based genetic profiles of small-subunit rRNA genes. Appl Environ Microbiol 66:930–936
Quaiser A, Ochsenreiter T, Lanz C, Schuster SC, Treusch AH, Eck J, Schleper C (2003) Acidobacteria form a coherent but highly diverse group within the bacterial domain: evidence from environmental genomics. Mol Microbiol 50:563–575
Ryckeboer J, Mergaert J, Vaes K, Klammer S, De Clercq D, Coosemans J, Insam H, Swings J (2003) A survey of bacteria and fungi occurring during composting and self-heating processes. Ann Microbiol 53:349–410
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schloss PD, Hay AG, Wilson DB, Walker LP (2003) Tracking temporal changes of bacterial community fingerprints during the initial stages of composting. FEMS Microbiol Ecol 46:1–9
Schmalenberger A, Tebbe CC (2003) Bacterial diversity in maize rhizospheres: conclusions on the use of genetic profiles based on PCR-amplified partial small subunit rRNA genes in ecological studies. Mol Ecol 12:251–261
Schmalenberger A, Schwieger F, Tebbe CC (2001) Effect of primers hybridizing to evolutionarily conserved regions of the small-subunit rRNA gene in PCR-based microbial community analyses and genetic profiling. Appl Environ Microbiol 67:3557–3563
Schwieger F, Tebbe CC (1998) A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl Environ Microbiol 64:4870–4876
Sekiguchi H, Tomioka N, Nakahara T, Uchiyama H (2001) A single band does not always represent single bacterial strains in denaturing gradient gel electrophoresis analysis. Biotechnol Lett 23:1205–1208
Steger K, Jarvis A, Smars S, Sundh I (2003) Comparison of signature lipid methods to determine microbial community structure in compost. J Microbiol Methods 55:371–382
Suzuki MT, Giovannoni SJ (1996) Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol 62:625–630
Tang JC, Inoue Y, Yasuta T, Yoshida S, Katayama A (2003) Chemical and microbial properties of various compost products. Soil Sci Plant Nutr 49:273–280
Tang JC, Kanamori T, Inoue Y, Yasuta T, Yoshida S, Katayama A (2004) Changes in the microbial community structure during thermophilic composting of manure as detected by the quinone profile method. Process Biochem 39:1999–2006
Tebbe CC (2002) Microbial genomes: DNA-based research uncovers composting microorganisms. Biocycle 43:24–27
Tebbe CC, Schmalenberger A, Peters S, Schwieger F (2001) Single-strand conformation polymorphism (SSCP) for microbial community analysis. In: Rochelle PA (ed) Environmental molecular microbiology: protocols and applications. Horizon, Wymondham, UK, pp 161–175
Tognetti C, Laos F, Mazzarino MJ, Hernandez MT (2005) Composting vs. vermicomposting: a comparison of end product quality. Compost Sci Util 13:6–13
Zhang H, Sekiguchi Y, Hanada S, Hugenholtz P, Kim H, Kamagata Y, Nakamura K (2003) Gemmatimonas aurantiaca gen. nov., sp nov., a gram-negative, aerobic, polyphosphate-accumulating micro-organism, the first cultured representative of the new bacterial phylum Gemmatimonadetes phyl. nov. Int J Syst Evol Microbiol 53:1155–1163
Zhang YQC, Ronimus RS, Turner N, Zhang Y, Morgan HW (2002) Enumeration of thermophilic Bacillus species in composts and identification with a random amplification polymorphic DNA (RAPD) protocol. Syst Appl Microbiol 25:618–626
Acknowledgements
We thank Karin Trescher for her excellent technical assistance. We also thank Alice B. Czarnetzki for helpful discussions, and the Marcopolo Environmental Group that funded part of the research and kindly provided compost materials for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fracchia, L., Dohrmann, A.B., Martinotti, M.G. et al. Bacterial diversity in a finished compost and vermicompost: differences revealed by cultivation-independent analyses of PCR-amplified 16S rRNA genes. Appl Microbiol Biotechnol 71, 942–952 (2006). https://doi.org/10.1007/s00253-005-0228-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0228-y