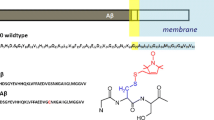
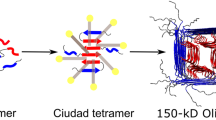
Abstract
Accumulating evidence suggests that Aβ1–42–membrane interactions may play an important role in the pathogenesis of Alzheimer’s disease. However, the mechanism of this structural transition remains unknown. In this work, we have shown that submicellar concentrations of sodium dodecyl sulfate (SDS) can provide a minimal platform for Aβ1–42 self-assembly. To further investigate the relation between Aβ1–42 structure and function, we analyzed peptide conformation and aggregation at various SDS concentrations using circular dichroism (CD), Fourier transform infrared spectroscopy, and gel electrophoresis. These aggregates, as observed via atomic force microscopy, appeared as globular particles in submicellar SDS with diameters of 35–60 nm. Upon sonication, these particles increased in disc diameter to 100 nm. Pyrene I 3/I 1 ratios and 1-anilinonaphthalene-8-sulfonic acid binding studies indicated that the peptide interior is more hydrophobic than the SDS micelle interior. We have also used Forster resonance energy transfer between N-terminal labeled pyrene and tyrosine (10) of Aβ1–42 in various SDS concentrations for conformational analysis. The results demonstrate that SDS at submicellar concentrations accelerates the formation of spherical aggregates, which act as niduses to form large spherical aggregates upon sonication.







Similar content being viewed by others
Abbreviations
- Aβ1–42 :
-
Amyloid β peptide, a 42 residue peptide
- AFM:
-
Atomic force microscopy
- ANS:
-
1-Anilinonapthalene-8-sulfonic acid ammonium salt
- CD:
-
Circular dichroism
- FRET:
-
Forster resonance energy transfer
- FTIR:
-
Fourier transformed infrared spectroscopy
- SDS:
-
Sodium dodecyl sulfate
- TFA:
-
Trifluoro acetic acid
- TFE:
-
2,2,2 Trifluoroethanol
References
Arispe N, Pollard HB, Rojas E (1993) Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein (A beta P-(1–40)) in bilayer membranes. Proc Natl Acad Sci USA 90:10573–10577. doi:10.1073/pnas.90.22.10573
Bokvist M, Lindstrom F, Watts A, Grobner G (2004) Two types of Alzheimer’s beta-amyloid (1–40) peptide membrane interactions: aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J Mol Biol 335:1039–1049. doi:10.1016/j.jmb.2003.11.046
Booth DR, Sunde M, Bellotti V, Robinson CV, Hutchinson WL, Fraser PE, Hawkins PN, Dobson CM, Radford SE, Blake CC, Pepys MB (1997) Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 385:787–793. doi:10.1038/385787a0
Chaney MO, Webster SD, Kuo YM, Roher AE (1998) Molecular modeling of the Abeta1-42 peptide from Alzheimer’s disease. Protein Eng 11:761–767. doi:10.1093/protein/11.9.761
Chen S, Berthelier V, Yang W, Wetzel R (2001) Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity. J Mol Biol 311:173–182. doi:10.1006/jmbi.2001.4850
Chiti F, Webster P, Taddei N, Clark A, Stefani M, Ramponi G, Dobson CM (1999) Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc Natl Acad Sci USA 96:3590–3594. doi:10.1073/pnas.96.7.3590
Choo LPI, Wetzel DL, Halliday W, Jackson M, LeVine SM, Mantsch HH (1996) In situ characterization of β-amyloid in Alzheimer’s diseased tissue by synchroton Fourier transform infrared microspectroscopy. Biophys J 71:1672–1679
Clark NS, Dodd I, Mossakowska DE, Smith RA, Gore MG (1996) Folding and conformational studies on SCR1-3 domains of human complement receptor 1. Protein Eng 9:877–884. doi:10.1093/protein/9.10.877
Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 8:79–84. doi:10.1038/nn1372
Coles M, Bicknell W, Watson AA, Fairlie DP, Craik DJ (1998) Solution structure of amyloid beta-peptide (1–40) in a water-micelle environment. Is the membrane-spanning domain where we think it is? Biochemistry 37:11064–11077. doi:10.1021/bi972979f
Crowther DC, Serpell LC, Dafforn TR, Gooptu B, Lomas DA (2003) Nucleation of α 1-antichymotrypsin polymerization. Biochemistry 42:2355–2363. doi:10.1021/bi0259305
Decker H, Ryan M, Jaenicke E, Terwilliger N (2001) SDS-induced phenoloxidase activity of hemocyanins from Limulus polyphemus, Eurypelma californicum, and Cancer magister. J Biol Chem 276:17796–17799. doi:10.1074/jbc.M010436200
Dong A, Prestrelski SJ, Allison SD, Carpenter JFJ (1995) Infrared spectroscopic studies of lyophilization- and temperature-induced protein aggregation. Pharm Sci 84:415–424
Ekiel I, Abrahamson M (1996) Folding-related dimerization of human cystatin C. J Biol Chem 271:1314–1321. doi:10.1074/jbc.271.3.1314
Fandrich M, Fletcher MA, Dobson CM (2001) Amyloid fibrils from muscle myoglobin. Nature 410:165–166. doi:10.1038/35065514
Fezoui Y, Teplow DB (2002) Kinetic studies of amyloid beta-protein fibril assembly. Differential effects of alpha-helix stabilization. J Biol Chem 277:36948–36954. doi:10.1074/jbc.M204168200
Finelli A, Kelkar A, Song HJ, Yang H, Konsolaki M (2004) A model for studying Alzheimer’s Abeta42-induced toxicity in Drosophila melanogaster. Mol Cell Neurosci 26:365–375. doi:10.1016/j.mcn.2004.03.001
Garavito RM, Ferguson-Miller SJ (2001) Detergents as tools in membrane biochemistry. J Biol Chem 276:32403–32406. doi:10.1074/jbc.R100031200
Gehman JD, O’Brien CC, Shabanpoor F, Wade JD, Separovic F (2008) Metal effects on the membrane interactions of amyloid-beta peptides. Eur Biophys J 37:333–344. doi:10.1007/s00249-007-0251-2
Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL (2003) Alzheimer’s disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA 18:10417–10422. doi:10.1073/pnas.1834302100
Guijarro JI, Sunde M, Jones JA, Campbell ID, Dobson CM (1998) Amyloid fibril formation by an SH3 domain. Proc Natl Acad Sci USA 95:4224–4228. doi:10.1073/pnas.95.8.4224
Halverson K, Fraser PE, Kirschner DA Jr, Lansbury PT (1990) Molecular determinants of amyloid deposition in Alzheimer’s disease: conformational studies of synthetic beta-protein fragments. Biochemistry 29:2639–2644. doi:10.1021/bi00463a003
Hatters DM, Lawrence LJ, Howlett GJ (2001) Submicellar phospholipid accelerates amyloid formation by apolipoprotein C-II. FEBS Lett 494:220–224. doi:10.1016/S0014-5793(01)02355-9
Jao CC, Der-Sarkissian A, Chen J, Langen R (2004) Structure of membrane-bound -synuclein studied by site-directed spin labeling. Proc Natl Acad Sci USA 101:8331–8336. doi:10.1073/pnas.0400553101
Jen CJ, Mclntire LV (1984) Characteristics of shear-induced aggregation in whole blood. J Lab Clin Med 103:115–124
Kagan BL, Hirakura Y, Azimov R, Azimova R, Lin MC (2002) The channel hypothesis of Alzheimer’s disease: current status. Peptides 23:1311–1315. doi:10.1016/S0196-9781(02)00067-0
Kakio A, Nishimoto S, Yanagisawa K, Kozutsumi Y, Matsuzaki K (2001) Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem 276:24985–24990. doi:10.1074/jbc.M100252200
Kayed R, Bernhagen J, Greenfield N, Sweimeh K, Brunner H, Voelter W, Kapurniotu AJ (1999) Conformational transitions of islet amyloid polypeptide (IAPP) in amyloid formation in vitro. Mol Biol 287:781–796. doi:10.1006/jmbi.1999.2646
Kelly JW (1998) The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr Opin Struct Biol 8:101–106. doi:10.1016/S0959-440X(98)80016-X
Kim J, Lee M (2004) Observation of multi-step conformation switching in β-amyloid peptide aggregation by fluorescence resonance energy transfer. Biochem Biophys Res Commun 316:393–397
Kirkitadze MD, Bitan G, Teplow DB (2002) Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: the emerging role of oligomeric assemblies. J Neurosci Res 69:567–577. doi:10.1002/jnr.10328
Kirkitadze MD, Condron MM, Teplow DB (2001) Identification and characterization of key kinetic intermediates in amyloid beta-protein fibrillogenesis. J Mol Biol 312:1103–1119. doi:10.1006/jmbi.2001.4970
Klein WL (2002) ADDLs & protofibrils—the missing links? Neurobiol Aging 23:231–235. doi:10.1016/S0197-4580(01)00312-8
Lai Z, Colon W, Kelly JW (1996) The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry 35:6470–6482. doi:10.1021/bi952501g
Lambert MP, Stevens G, Sabo S, Barber K, Wang G, Wade W, Krafft G, Snyder S, Holzman TF, Klein WL (1994) Beta/A4-evoked degeneration of differentiated SH-SY5Y human neuroblastoma cells. J Neurosci Res 39:377–385. doi:10.1002/jnr.490390404
Le Berre F, Chauveteau G, Pefferkorn EJ (1998) Shear induced aggregation/fragmentation of hydrated colloids. Colloid Interface Sci 199:13–21. doi:10.1006/jcis.1997.5308
Lopez De La Paz M, Goldie K, Zurdo J, Lacroix E, Dobson CM, Hoenger H, Serrano L (2002) De novo designed peptide-based amyloid fibrils. Proc Natl Acad Sci USA 99:16052–16057. doi:10.1073/pnas.252340199
Lougheed WD, Woutfe-Flanagan H, Clement JR, Albisser AM (1980) Insulin aggregation in artificial delivery systems. Diabetologia 19:1–9. doi:10.1007/BF00258302
Ma K, Talafous J, Orlando R, Zagorski MG (1997) Trifluoroacetic acid pretreatment reproducibly disaggregates the amyloid β-peptide. Int J Exp Clin Invest 4:240–252
Mark A Thompson, Planaria Software LLC, Seattle, WA http://www.arguslab.com
Matsunaga Y, Ierovnik E, Yamada T, Turk V (2002) Conformational changes preceding amyloid-fibril formation of amyloid-beta and stefin B; parallels in pH dependence. Curr Med Chem 9:1717–1724
Morillas M, Swietnicki W, Gambetti P, Surewicz WKJ (1999) Membrane environment alters the conformational structure of the recombinant human prion protein. J Biol Chem 274:36859–36865. doi:10.1074/jbc.274.52.36859
Motta A, Andreotti G, Amodeo P, Strazzullo G, Castiglione Morelli MA (1998) Solution structure of human calcitonin in membrane-mimetic environment: the role of the amphipathic helix. Proteins 32:314–323. doi:10.1002/(SICI)1097-0134(19980815)32:3<314::AID-PROT7>3.0.CO;2-H
Muga A, Arrondo JLR, Bellon T, Sancho J, Bernabeu C (1993) Arch Biochem Biophys 300:451–457. doi:10.1006/abbi.1993.1061
Narayanaswami V, Kim J, McNamee MG (1993) Protein–lipid interactions and Torpedo californica nicotinic acetylcholine receptor function. 1. Spatial disposition of cysteine residues in the gamma subunit analyzed by fluorescence-quenching and energy-transfer measurements. Biochemistry 32:12413–12419. doi:10.1021/bi00097a020
Narayanaswami V, Szeto S, Ryan RO (2001) Lipid-association induced N- and C-terminal domain reorganization in human apolipoprotein E3. J Biol Chem 276:37853–37860
O’Nuallain B, Williams AD, Westermark P, Wetzel R (2004) Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem 279:17490–17499. doi:10.1074/jbc.M311300200
Pertinhez TA, Bouchard M, Smith RA, Dobson CM, Smith LJ (2002) Stimulation and inhibition of fibril formation by a peptide in the presence of different concentrations of SDS. FEBS Lett 529:193–197. doi:10.1016/S0014-5793(02)03333-1
Prusiner SB (1997) Prion diseases and the BSE crisis. Science 278:245–251. doi:10.1126/science.278.5336.245
Ramirez-Alvarado M, Merkel JS, Regan L (2000) A systematic exploration of the influence of the protein stability on amyloid fibril formation in vitro. Proc Natl Acad Sci USA 97:8979–8984. doi:10.1073/pnas.150091797
Rangachari V, Reed DK, Moore DB, Rosenberry TL (2006) Secondary structure and interfacial aggregation of amyloid-β(1–40). Biochemistry 45:8639–8648. doi:10.1021/bi060323t
Rangachari V, Moore DB, Reed DK, Sonoda LK, Bridges AW, Conboy E, Hartigan D, Rosenberry TL (2007) Amyloid-beta(1–42) rapidly forms protofibrils and oligomers by distinct pathways in low concentrations of sodium dodecylsulfate. Biochemistry 46:12451–12462. doi:10.1021/bi701213s
Rochet JC Jr, Lansbury PT (2000) Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol 10:60–68. doi:10.1016/S0959-440X(99)00049-4
Satheeshkumar KS, Jayakumar R (2002) Sonication induced sheet formation at the air–water interface. Chem Commun (Camb) 7:2244–2245. doi:10.1039/b206886a
Schwyzer R (1986) Molecular mechanism of opioid receptor selection. Biochemistry 25:6335–6342. doi:10.1021/bi00368a075
Selkoe DJ (1996) Amyloid β-protein and the genetics of Alzheimer’s disease. J Biol Chem 271:18295–18298
Shoa H, Jao S, Ma K, Zagorski MG (1999) Solution structure of micelle-bound amyloid β-(1-40) and β-(1-42) peptides of alzheimer’s disease. J Mol Biol 285:755–773. doi:10.1006/jmbi.1998.2348
Solomon B (2002) Mini Rev. Towards Alzheimer’s disease vaccination. Med Chem 2:85–92
Soto C (2001) Protein misfolding and disease; protein refolding and therapy. FEBS Lett 498:204–207. doi:10.1016/S0014-5793(01)02486-3
Sreerama N, Woody RW (2000) Anal. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Biochemistry 287:252–260
Stathopulos PB, Scholz GA, Hwang Y-M, Rumfeldt JAO, Leprock JR, Meiering EM (2004) Sonication of protein causes formation of aggregates that resemble amyloid. Protein Sci 13:1–11. doi:10.1110/ps.03309504
Stryer AL (1978) Fluorescence energy transfer as a spectroscopic ruler. Rev Biochem 47:819–846. doi:10.1146/annurev.bi.47.070178.004131
Surewicz WK, Mantsch HH, Chapman D (1993) Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry 32:389–394. doi:10.1021/bi00053a001
Terzi E, Holzemann G, Seelig J (1995) Self-association of beta-amyloid peptide (1–40) in solution and binding to lipid membranes. J Mol Biol 252:633–642. doi:10.1006/jmbi.1995.0525
Tew DJ, Bottomley SP, Smith DP, Ciccotosto GD, Babon J, Hinds MG, Masters CL, Cappai R, Barnham KJ (2008) Stabilization of neurotoxic soluble beta-sheet-rich conformations of the Alzheimer’s disease amyloid-beta peptide. Biophys J 94:2752–2766. doi:10.1529/biophysj.107.119909
Uversky VN, Fink AL (2004) Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta 1698:131–153
Uversky VN, Li J, Fink AL (2001) Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem 276:10737–10744. doi:10.1074/jbc.M010907200
Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ (2002) Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans 30:552–557. doi:10.1042/BST0300552
Waterhous DV Jr, Johnson WC (1994) Importance of environment in determining secondary structure in proteins. Biochemistry 33:2121–2128. doi:10.1021/bi00174a019
Wu P, Li YK, Talalay P, Brand L (1994) Characterization of the three-tyrosine residues of delta 5–3-ketosteroid isomerase by time-resolved fluorescence and circular dichroism. Biochemistry 33:7415–7422. doi:10.1021/bi00189a048
Yamamoto S, Hasegawa K, Yamaguchi I, Tsutsumi S, Kardos J, Goto Y, Gejyo F, Naiki H (2004) Low concentrations of sodium dodecyl sulphate induce the extension of β-microglobulin-related amyloid fibrils at a neutral pH. Biochemistry 43:11075–11082. doi:10.1021/bi049262u
Acknowledgments
We thank Dr. T. Ramasami, Director, CLRI, for his kind support for this work. One of the author, N. Sureshbabu thanks the Council for Scientific and Industrial Research (CSIR) for providing funds in the form of SRF. We also thank Dr. Ganesh, Vanderbilt University, for his help with peptide synthesis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sureshbabu, N., Kirubagaran, R. & Jayakumar, R. Surfactant-induced conformational transition of amyloid β-peptide. Eur Biophys J 38, 355–367 (2009). https://doi.org/10.1007/s00249-008-0379-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-008-0379-8