Abstract
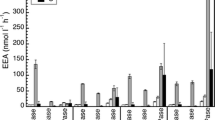
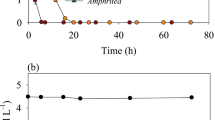
We determined the total and dissolved extracellular enzymatic activity (EEA) of α-glucosidase and β-glucosidase (AGase and BGase), alkaline phosphatase (APase) and leucine aminopeptidase (LAPase) activities in the epi-, meso- and bathypelagic waters of the subtropical Northeast Atlantic. EEA was also determined in treatments in which bacterial EEA was inhibited by erythromycin. Additionally, EEA decay experiments were performed with surface and deep waters to determine EEA lifetimes in both water masses. The proportion of dissolved to total EEA (66–89 %, 44–88 %, 57–82 % and 86–100 % for AGase, BGase, APase and LAPase, respectively) was generally higher than the cell-associated (i.e., particulate) EEA. The percentage of dissolved to total EEA was inversely proportional to the percentage of erythromycin-inhibited to total EEA. Since erythromycin-inhibited plus dissolved EEA equaled total EEA, this tentatively suggests that cell-associated EEA in the open oceanic water column is almost exclusively of bacterial origin. The decay constants of dissolved EEA were in the range of 0.002–0.048 h−1 depending on the type of extracellular enzyme, temperature and depth in the water column. Although dissolved EEA can have different origins, the major contribution of Bacteria to cell-associated EEA and the long life-time of dissolved EEA suggest that Bacteria—and not mesophilic Archaea—are essentially the main producers of EEA in the open subtropical Northeast Atlantic down to bathypelagic layers.




Similar content being viewed by others
References
Azam F, Cho BC (1987) Bacterial utilization of organic matter in the sea. Ecology of microbial communities. Cambridge University Press, Cambridge, pp 261–281
Amon RMW, Benner R (1996) Bacterial utilization of different size classes of dissolved organic matter. Limnol Oceanogr 41:41–51
Weiss M, Abele U, Weckesser J, Welte W, Schiltz E, Schulz GE (1991) Molecular architecture and electrostatic properties of bacterial porin. Science 254:1627–1630
Hoppe H-G, Arnosti C, Herndl GJ (2002) Ecological significance of bacterial enzymes in the marine environment. In: Burns RG, Dick RP (eds) Enzymes in the environment: activity, ecology, and applications. Marcel Dekker, New York, pp 73–108
Hoppe H-G (1983) Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates. Mar Ecol Prog Ser 11:299–308
Someville M, Billen G (1983) A method for determining exoproteolytic activity in natural waters. Limnol Oceanogr 28:190–193
Chrost RJ, Rai H (1993) Ectoenzyme activity and bacterial secondary production in nutrient-improverished and nutrient-enriched mesocosms. Microb Ecol 25:131–150
Rego JV, Billen G, Fontigny A, Someville M (1985) Free and attached proteolytic activity in water environments. Mar Ecol Prog Ser 21:245–249
Duhamel S, Dyhrman ST, Karl DM (2010) Alkaline phosphatase activity and regulation in the North Pacific Subtropical Gyre. Limnol Oceanogr 55:1414–1425
Baltar F, Arístegui J, Gasol JM, Sintes E, Aken HM, Herndl GJ (2010) High dissolved extracellular enzymatic activity in the deep central Atlantic Ocean. Aquat Microb Ecol 58:287–302
Karner MB, DeLong EF, Karl DM (2001) Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507–510
Teira E, Lebaron P, Hv A, Herndl GJ (2006) Distribution and activity of Bacteria and Archaea in the deep water masses of the North Atlantic. Limnol Oceanogr 51:2131–2144
Kirchman DL, Elifantz H, Dittel AI, Malmstrom RR, Cottrell MT (2007) Standing stock and activity of Archaea and Bacteria in the western Arctic Ocean. Limnol Oceanogr 52:495–507
Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (2008) Mesophilic Crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol 6:245–252
Varela M, van Aken HM, Sintes E, Herndl GJ (2008) Latitudinal trends of Crenarchaeota and Bacteria in the meso- and bathypelagic water masses of the Eastern North Atlantic. Environ Microbiol 10:110–124
Hansman RL, Griffin S, Watson JT, Druffel ERM, Ingalls AE (2009) The radiocarbon signature of microorganisms in the mesopelagic ocean. Proc Natl Acad Sci 106:6513–6518
Yokokawa T, Olbrich K, Sintes E, Herndl GJ (in press) Heterotrophic activity of Archaea and Bacteria througout the water column of the eastern Atlantic. Aquat Microb Ecol
Gasol JM, del Giorgio PA (2000) Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci Mar 64:197–224
Baltar F, Sintes E, Van Aken H, Gasol JM, Arístegui J, Herndl GJ (2009) Prokaryotic extracellular enzymatic activity in relation to biomass production and respiration in the meso- and bathypelagic waters of the (sub)tropical Atlantic. Environ Microbiol 11:1998–2014
Hoppe HG (1993) Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria. Handbook of methods in aquatic microbial ecology, pp 423–431
Azúa I, Uanue M, Ayo B, Arrtolozaga I, Arrieta JM, Iriberri J (2003) Influence of organic matter quality in the cleavage of polymers by marine bacterial communities. J Plankton Res 25:1451–1460
Unanue M, Ayo B, Agis M, Slezak D, Herndl GJ, Iriberri J (1999) Ectoenzymatic activity and uptake of monomers in marine bacterioplankton described by a biphasic kinetic model. Microb Ecol 37:36–48
Kim C, Nishimura Y, Nagata T (2007) High potential activity of alkaline phosphatase in the benthic nepheloid layer of a large mesotrophic lake: implications for phosphorus regeneration in oxygenated hypolimnion. Aquat Microb Ecol 49:303–311
Nagata T, Kirchman DL (1990) Filtration-induced release of dissolved free amino acids: application to cultures of marine protozoa. Mar Ecol Prog Ser 68:1–5
Obayashi Y, Suzuki S (2008) Adsorption of extracellular proteases in seawater onto filters during size fractionation. J Oceanogr 64:367–372
Katzung B (2003) Basic and clinical pharmacology. McGraw Hill, New York
Hoppe HG (2003) Phosphatase activity in the sea. Hydrobiologia 493:187–200
Chrost RJ (1991) Microbial enzymes in aquatic environments. Springer Verlag, New York
Zaccone R, Boldrin A, Caruso G, La Ferla R, Maimone G, Santinelli C, Turchetto M (2012) Enzymatic activities and prokaryotic abundance in relation to organic matter along a west–east Mediterranean Transect (TRANSMED Cruise). Microb Ecol 64:1–13
Hoppe H-G, Ullrich S (1999) Profiles of ectoenzymes in the Indian Ocean: phenomena of phosphatase activity in the mesopelagic zone. Aquat Microb Ecol 19:139–148
Koike I, Nagata T (1997) High potential activity of extracellular alkaline phosphatase in deep waters of the central Pacific. Deep-Sea Res 44:2283–2294
Azzaro M, La Ferla R, Maimone G, Monticelli L, Zaccone R, Civitarese G (2011) Prokaryotic dynamics and heterotrophic metabolism in a deep convection site of Eastern Mediterranean Sea (the Southern Adriatic Pit). Cont Shelf Res 44:106–118
Zaccone R, Monticelli LS, Seritti A, Santinelli C, Azzaro M, Boldrin A, LaFerla R, D’Alcala MR (2003) Bacterial processes in the intermediate and deep layers of the Ionian Sea in winter 1999: vertical profiles and their relationship to the different water masses. J Geophys Res 108:8117
Tamburini C, Garel M, Ali BA, Mérigot B, Kriwy P, Charrière B, Budillon G (2009) Distribution and activity of Bacteria and Archaea in the different water masses of the Tyrrhenian Sea. Deep-Sea Res Part II 56:700–712
Tamburini C, Garcin J, Ragot M, Bianchi A (2002) Biopolymer hydrolysis and bacterial production under ambient hydrostatic pressure through a 2000 m water column in the NW Mediterranean. Deep Sea Res II 49:2109–2123
Celussi M, Cataletto B, Umani CF, Negro PD (2009) Depth profile of bacterioplankton assemblages and their activities in two different areas of the Ross Sea (Antarctica). Deep-Sea Res I 56:2193–2205
Hashimoto S, Fujiwara K, Fuwa K, Saino T (1985) Distribution and characteristics of carboxypeptidase activity in pond, river, and seawater in the vicinity of Tokyo. Limnol Oceanogr 30:631–645
Karner M, Rassoulzadegan F (1995) Extracellular enzyme activity: indications for high short-term variability in a coastal marine ecosystem. Microb Ecol 30:143–156
Keith SC, Arnosti C (2001) Extracellular enzyme activity in a river–bay–shelf transect: variations in polysaccharide hydrolysis rates with substrate and size class. Aquat Microb Ecol 24:243–253
Karrasch B, Ullrich S, Mehrens M, Zimmermann-Timm H (2003) Free and particle-associated extracellular enzyme activity and bacterial production in the lower Elbe estuary, Germany. Acta Hydroch Hydrob 31:297–306
Ziervogel K, Arnosti C (2008) Polysaccharide hydrolysis in aggregates and free enzyme activity in aggregate-free seawater from the north-eastern Gulf of Mexico. Environ Microbiol 10:289–299
Ziervogel K, Steen AD, Arnosti C (2010) Changes in the spectrum and rates of extracellular enzyme activities in seawater following aggregate formation. Biogeosciences 7:1007–1015
Davey KE, Kirby RR, Turley CM, Weightman AJ, Fry JC (2001) Depth variation of bacterial extracellular enzyme activity and population diversity in the northeastern North Atlantic Ocean. Deep Sea Res II 48:1003–1017
Agogué H, Brink M, Dinasquet J, Herndl GJ (2008) Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic. Nature 456:788–791
Alderkamp AC, Mv R, Bolhuis H (2007) Characterization of marine bacteria and the activity of their enzyme systems involved in degradation of the algal storage glucan laminarin. FEMS Microbiol Ecol 59:108–117
Albertson NH, Nystrom T, Kjelleberg S (1990) Macromolecular-synthesis during recovery of the marine Vibrio sp. S14 from starvation. J Gen Microbiol 136:2201–2207
Chrost RJ (1991) Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. Microbial enzymes in aquatic environments. Springer Verlag, New York, pp 29–59
Antranikian G (1992) Microbial degradation of starch. In: Wilkelman G (ed) Microbial Degradation of Natural Products. John Wiley & Sons Weinheim, Germany, pp 105–115
Bochdansky AB, Puskaric S, Herndl GJ (1995) Influence of zooplankton grazing on free dissolved enzymes in the sea. Mar Ecol Prog Ser 121:53–63
Li H, Veldhuis MJW, Post AF (1998) Alkaline phosphatase activities among planktonic communities in the northern Red Sea. Mar Ecol Prog Ser 173:107–115
Steen AD, Arnosti C (2011) Long lifetimes of beta-glucosidase, leucine aminopeptidase, and phosphatase in Arctic seawater. Mar Chem 123:127–132
Smith DC, Simon M, Alldredge AL, Azam F (1992) Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature 359:139–142
Gianfreda L, Scarfi MR (1991) Enzyme stabilization: state of the art. Molecular and Cellular Biochemistry 100:97–128
Naidja A, Huang PM, Bollag JM (2000) Enzyme–clay interactions and their impact on transformations of natural and anthropogenic organic compounds in soil. J Environ Qual 29:677–691
Ziervogel K, Karlsson E, Arnosti C (2007) Surface associations of enzymes and of organic matter: consequences for hydrolytic activity and organic matter remineralization in marinne systems. Mar Chem 104:241–252
Lähdesmäki P, Piispanen R (1992) Soil enzymology: role of protective colloid systems in the preservation of exoenzyme activities in soil. Soil Biol Biochem 24:1173–1177
Lozzi I, Calami L, Fusi P, Bosetto M, Stotzky G (2001) Interaction of horseradish peroxidase with montmorillonite homoionic to Na+ and Ca2+: effects on enzymatic activity and microbial degradation. Soil Biol Biochem 33:1021–1028
Decho AW (1990) Microbial exopolymers secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr Mar Biol Ann Rev 28:73–153
Chrost JR (1991) Microbial enzymes in aquatic environments. Springer, New York, 1991
Nagata T, Kirchman DL (1992) Release of macromolecular organic complexes by heterotrophic marinen flagellates. Mar Ecol Prog Ser 83:233–240
Verdugo P, Orellana MV, Chin WC, Petersen TW, Gvd E, Benner R, Hedges JI (2008) Marine biopolymer self-assembly: implications for carbon cycling in the ocean. Royal Soc Chem 139:393–398
Baltar F, Arístegui J, Gasol JM, Sintes E, Herndl GJ (2009) Evidence of prokaryotic metabolism on suspended particulate organic matter in the dark waters of the subtropical North Atlantic. Limnol Oceanogr 54:182–193
Baltar F, Arístegui J, Sintes E, Gasol JM, Reinthaler T, Herndl GJ (2010) Significance of non-sinking particulate organic carbon and dark CO2 fixation to heterotrophic carbon demand in the mesopelagic northeast Atlantic. Geophys Res Lett 37:L09602. doi:09610.01029/02010GL043105
Antia AN, Koeve W, Fischer G, Blanz T, Schulz-Bull D, Scholten J, Neuer S, Kremling K, Kuss J, Peinert R, Hebbeln D, Bathmann U, Conte M, Fehner U, Zeitzschel B (2001) Basin-wide particulate carbon flux in the Atlantic Ocean: regional export patterns and potential for atmospheric CO2 sequestration. Global Biogeochem Cycles 15:845–862
Bochdansky AB, Aken HM, Herndl GJ (2010) Role of macroscopic particles in deep-sea oxygen consumption. Proc Natl Acad Sci 107:8287–8291
DeLong EF, Preston CM, Mincer T, Rich V, Hallam SJ, Frigaard NU, Martinez A, Sullivan MB, Edwards R, Brito BR, Chisholm SW, Karl DM (2006) Community genomics among stratified microbial assemblages in the ocean’s interior. Science 311:496–503
Allison SD (2005) Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol Lett 8:626–635
Vetter YA, Deming JW, Jumars PA, Krieger-Brockett BB (1998) A predicitive model of bacterial foraging by means of freely released extracellular enzymes. Microb Ecol 36:75–92
Reinthaler T, Aken H, Veth C, Williams PJ, Aristegui J, Robinson C, Lebaron P, Herndl GJ (2006) Prokaryotic respiration and production in the meso- and bathypelagic realm of the eastern and western North Atlantic basin. Limnol Oceanogr 51:1262–1273
Gasol JM, Alonso-Sáez L, Vaqué D, Baltar F, Calleja ML, Duarte CM, Arístegui J (2009) Mesopelagic prokaryotic bulk and single-cell heterotrophic and community composition in the NW Africa–Canary Islands coastal-transition zone. Prog Oceanogr 83:189–196
Baltar F, Arístegui J, Gasol JM, Herndl GJ (2012) Microbial functioning and community structure variability in the mesopelagic and epipelagic waters of the subtropical NE Atlantic. Appl Environ Microbiol 78:3309–3316
Baltar F, Arístegui J, Gasol JM, Lekunberri I, Herndl GJ (2010) Mesoscale eddies: hotspots of prokaryotic activity and differential community structure in the ocean. ISME J 4:975–988
Acknowledgments
This research was carried out in the frame of the IMBER-endorsed Spanish project CAIBEX (CTM2007-66408-C02-02) “Plan Nacional de I + D” (MEC), coordinated by J.A. Partial support was obtained by a grant of the Earth and Life Science Division of the Dutch Science Foundation (ALW-NWO; ARCHIMEDES project, 835.20.023) and the ESF MOCA project and the Austrian Science Fund (FWF) projects: I486-B09 and P23234-B11 to G.J.H., by the project STORM (CTM2009-09352/MAR) to J.M.G., a predoctoral Fellowship of the Spanish Ministry of Education and Science (AP2005-3932) and a postdoctoral grant under the MOCA (Microbial Oceanography of ChemolithoAutotrophic planktonic Communities; ESF – Eurocores Program Evolutionary and Ecological Functional Genomics) project to F.B. We thank the captain and crew of the R/V Sarmiento de Gamboa for their support at sea, and M.F. Montero for the flow-cytometry analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baltar, F., Arístegui, J., Gasol, J.M. et al. Bacterial Versus Archaeal Origin of Extracellular Enzymatic Activity in the Northeast Atlantic Deep Waters. Microb Ecol 65, 277–288 (2013). https://doi.org/10.1007/s00248-012-0126-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0126-7