Abstract
Background
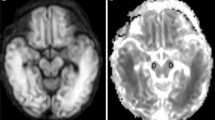
Diffusion-weighted imaging is a valuable tool in the assessment of the neonatal brain, and changes in diffusion are seen in normal development as well as in pathological states such as hypoxic–ischemic encephalopathy (HIE). Various methods of quantitative assessment of diffusion values have been reported. Global ischemic injury occurring during the time of rapid developmental changes in brain myelination can complicate the imaging diagnosis of neonatal HIE.
Objective
To compare a quantitative method of histographic analysis of brain apparent coefficient (ADC) maps to the qualitative interpretation of routine brain MR imaging studies. We correlate changes in diffusion values with gestational age in radiographically normal neonates, and we investigate the sensitivity of the method as a quantitative measure of hypoxic–ischemic encephalopathy.
Materials and methods
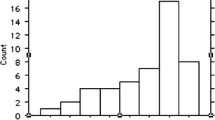
We reviewed all brain MRI studies from the neonatal intensive care unit (NICU) at our university medical center over a 4-year period to identify cases that were radiographically normal (23 cases) and those with diffuse, global hypoxic–ischemic encephalopathy (12 cases). We histographically displayed ADC values of a single brain slice at the level of the basal ganglia and correlated peak (s-sDav) and lowest histogram values (s-sDlowest) with gestational age.
Results
Normative s-sDav values correlated significantly with gestational age and declined linearly through the neonatal period (r 2 = 0.477, P < 0.01). Six of 12 cases of known HIE demonstrated significantly lower s-sDav and s-sDlowest ADC values than were reflected in the normative distribution; several cases of HIE fell within a 95% confidence interval for normative studies, and one case demonstrated higher-than-normal s-sDav.
Conclusion
Single-slice histographic display of ADC values is a rapid and clinically feasible method of quantitative analysis of diffusion. In this study normative values derived from consecutive neonates without radiographic evidence of ischemic injury are correlated with gestational age, declining linearly throughout the perinatal period. This method does identify cases of HIE, though the overall sensitivity of the method is low.





Similar content being viewed by others
References
Barkovich AJ (2005) Brain and spine injuries in infancy and childhood. Pediatric neuroimaging. Lippincott, Williams and Wilkins, Philadelphia, pp 190–290
Heinz ER, Provenzale JM (2009) Imaging findings in neonatal hypoxia: a practical review. AJR Am J Roentgenol 192:41–47
Rutherford M, Srinivasan L, Dyet L et al (2006) Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol 36:582–592
Alderliesten T, de Vries LS, Benders MJ et al (2011) MR imaging and outcome of term neonates with perinatal asphyxia: value of diffusion-weighted MR imaging and (1)H MR spectroscopy. Radiology 261:235–242
Barkovich AJ, Miller SP, Bartha A et al (2006) MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol 27:533–547
Hunt RW, Neil JJ, Coleman LT et al (2004) Apparent diffusion coefficient in the posterior limb of the internal capsule predicts outcome after perinatal asphyxia. Pediatrics 114:999–1003
Liauw L, van Wezel-Meijler G, Veen S et al (2009) Do apparent diffusion coefficient measurements predict outcome in children with neonatal hypoxic–ischemic encephalopathy? AJNR Am J Neuroradiol 30:264–270
Okereafor A, Allsop J, Counsell SJ et al (2008) Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 121:906–914
Chun T, Filippi CG, Zimmerman RD et al (2000) Diffusion changes in the aging human brain. AJNR Am J Neuroradiol 21:1078–1083
Rutherford M, Ramenghi LA, Edwards AD et al (2010) Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic–ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 9:39–45
Azzopardi DV, Strohm B, Edwards AD et al (2009) Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 361:1349–1358
Shankaran S, Laptook AR, Ehrenkranz RA et al (2005) Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N Engl J Med 353:1574–1584
Fisher M, Bogousslavsky J (1993) Evolving toward effective therapy for acute ischemic stroke. JAMA 270:360–364
Grotta J, Clark W, Coull B et al (1995) Safety and tolerability of the glutamate antagonist CGS 19755 (Selfotel) in patients with acute ischemic stroke. Results of a phase IIa randomized trial. Stroke 26:602–605
Ulug AM (2002) Monitoring brain development with quantitative diffusion tensor imaging. Dev Sci 5:286–292
Zhang L, Thomas KM, Davidson MC et al (2005) MR quantitation of volume and diffusion changes in the developing brain. AJNR Am J Neuroradiol 26:45–49
Lovblad KO, Schneider J, Ruoss K et al (2003) Isotropic apparent diffusion coefficient mapping of postnatal cerebral development. Neuroradiology 45:400–403
Provenzale JM, Isaacson J, Chen S et al (2010) Correlation of apparent diffusion coefficient and fractional anisotropy values in the developing infant brain. AJR Am J Roentgenol 195:W456–W462
Neil JJ, Shiran SI, McKinstry RC et al (1998) Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 209:57–66
McKinstry RC, Miller JH, Snyder AZ et al (2002) A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 59:824–833
Toft PB, Leth H, Peitersen B et al (1996) The apparent diffusion coefficient of water in gray and white matter of the infant brain. J Comput Assist Tomogr 20:1006–1011
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cauley, K.A., Filippi, C.G. Apparent diffusion coefficient histogram analysis of neonatal hypoxic–ischemic encephalopathy. Pediatr Radiol 44, 738–746 (2014). https://doi.org/10.1007/s00247-013-2864-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-013-2864-1