Abstract
Chronic right ventricular (RV) pacing can induce structural and functional cardiac deterioration. Because animal studies showed a benefit of left ventricular (LV) over RV pacing, this study compared the effects of chronic RV and LV pacing in children. Retrospectively, echocardiographic data were evaluated from 18 healthy children (control subjects) and from children undergoing chronic epicardial RV pacing (7 RVP) or LV pacing (7 LVP). Assessment included LV end-diastolic wall thickness (LVEDWT) and end-systolic wall thickness (LVESWT) as well as LV end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD). The shortening fraction and eccentricity index (LV diameter/2 × LV wall thickness) were calculated as measures of LV function and eccentricity, respectively. Duration of QRS and septal posterior wall motion delay (SPWMD) were used as measures of electrical and mechanical dyssynchrony, respectively. A p value less than 0.05 determined significance. As the findings showed, LVEDD, LVESD, LVEDWT, and LVESWT were not significantly different between the groups. The shortening fraction was significantly lower in the RVP (21.7% ± 6.0%) than in the LVP (32.2% ± 5.2%) or control (29.3% ± 4.3%) children. The systolic LV eccentricity index was significantly larger in the RVP (1.8 ± 0.2) than in the LVP (1.4 ± 0.1) or control (1.4 ± 0.2) children. The SPWMD was significantly larger in the RVP (338 ± 20 ms) than in the LVP (−16 ± 14 ms) or control (−5 ± 35 ms) group, whereas QRS duration was similarly longer in the RVP (157 ± 10 ms) and LVP (158 ± 22 ms) groups compared than in the control group (69 ± 7 ms). The authors conclude that LV function in children is preserved by chronic pacing at the LV lateral wall.
Similar content being viewed by others
In children and adults with congenital or acquired atrioventricular (AV) block, the ventricular pacing lead is traditionally positioned at the right ventricle (RV) [16, 19]. However, RV apex pacing causes an acute decrease in left ventricular (LV) function in animals [26], adults [6] and children [14, 36]. During chronic RV pacing in children, LV function, morphology [30, 31], and histology [15] are at risk for deterioration over time (for review see Karpawich [16]). Chronic RV pacing can eventually result in cardiac failure, which occurs in 6% to 7% of children [17, 20, 38]. Also, in adults, chronic RV apex pacing has deleterious effects (for review see Manolis [19]) and increases the risk of heart failure [1, 28].
Recognition of the possible harmful effects from RV apex pacing initiated the search for alternative ventricular pacing sites including the RV outflow tract, His bundle, LV wall, and biventricular pacing. Pacing at the His bundle is likely the superior approach [8], but appears to be technically difficult, especially in children. Right ventricle outflow tract pacing does not provide a consistent beneficial hemodynamic effect compared with RV apex pacing [6]. During pacing at different RV septal sites, hemodynamic function varies widely, and the location of the RV septal site that leads to the less pronounced decrease in pump function also varies between hearts in canine experiments [22].
In adults with heart failure, both LV lateral wall pacing [3, 27] and biventricular (RV apex + LV lateral wall) pacing [3] acutely result in a better functional outcome than RV apex pacing. Also in adults, LV pacing alone seems to be as effective as biventricular resynchronization therapy, both in the acute situation [21] and after 6 months of pacing [33].
The current study aimed to investigate the long-term functional and structural outcome of epicardial RV and LV pacing in children. The study was performed retrospectively using echocardiographic and electrocardiographic (ECG) data from children undergoing chronic RV or LV pacing and from healthy children (control subjects).
Methods
Study Population and Pacing Characteristics
All children with normal cardiac anatomy and a ventricular pacemaker in the database of the Children’s University Hospital in Zurich (Switzerland) were considered for inclusion in this study. The study included all children with congenital or acquired AV block (except for cardiomyopathy) undergoing chronic epicardial RV or LV pacing for rate control (minimum of 95% paced beats) for whom echocardiographic and ECG data were available.
Data were acquired during the most recent outpatient clinic visit of children with chronic RV pacing (RVP, n = 7) or LV pacing (LVP, n = 7) and evaluated retrospectively. For the children who underwent a pacing lead replacement, the last echocardiography and the ECG data before the replacement were evaluated to exclude the effects of changes in the pacing site between the initiation of pacing and the moment the data were obtained. In addition, for a small number of patients (3 RVP and 4 LVP), the echocardiographic preimplantation data were of sufficient quality for assessment and are presented to provide an estimation of the baseline characteristics.
Bipolar steroid-eluting pacing leads (Medtronic CapSure Epi 10366 or 4968; Medtronic Inc., Minneapolis, MN, USA) were implanted in all children and connected to various pulse generators. Pacemaker lead positioning was purely determined by the surgical approach preferred by surgeons [9]. Through a sternotomy or using a subxyphoidal approach, RV pacing leads were implanted and positioned at the RV apex (n = 6) or RV free wall (n = 1). Left ventricular pacing leads were implanted through a left lateral thoracotomy and placed at the LV mid lateral wall [9].
Table 1 depicts the characteristics of the paced children. Before pacemaker implantation and during the follow-up period, none of the patients had clinical symptoms of heart failure, received any cardiovascular drugs, or had echocardiographic signs of cardiac failure. The control group consisted of 18 healthy children who visited the outpatient clinic for an innocent cardiac murmur. This retrospective study was approved by the local ethics committee and performed according to their guidelines.
Echocardiographic Evaluation
Echocardiographic data were obtained in the standard precordial positions with appropriate transducers (7.5-, 5.0-, 3.5-, and 2.5-MHz; Vivid 7; General Electric Healthcare, UK; or Philips Sonos 5500; Philips, Best, The Netherlands). Data were stored either digitally or on VHS videotapes, then subsequently digitized offline. End-diastolic and end-systolic frames of the parasternal short-axis views were further analyzed using Matlab software (The Mathworks Inc., Natick, MA, USA).
Two observers, blinded to the study group of the patient, each performed three independent measurements in random order for every patient. The average of the measurements performed by the two observers was used for further analysis. A representative example of a processed echocardiographic image is depicted in Fig. 1.
Body surface area was calculated using the formula of Du Bois and Du Bois [10]:
Left ventricular end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD) were measured bidirectionally (Fig. 1) and averaged to minimize over- or underestimation in case of an asymmetric mechanical activation pattern. To estimate the degree of LV dilation and to compare patients of different ages and weights, LVEDD was expressed as a z-score of normal [5].
Shortening fraction (SF) as a measure of cardiac function was defined as:
Regional changes in LV wall thickness were assessed by measuring the LV wall in six consecutive regions at end-diastole and end-systole on short-axis views (Fig. 1). The mean of these six regions was used as the mean LV end-diastolic wall thickness (LVEDWT) and the mean LV end-systolic wall thickness (LVESWT). Again, LVEDWT was expressed as a z-score of normal.
The eccentricity index of the LV (as a measure of fiber stress) was calculated as follows:
Septal posterior wall motion delay (SPWMD), as a measure of intraventricular mechanical synchrony, was obtained from short-axis M-mode echocardiographic images and defined as the time delay between the earliest peak of systolic inward movement of the septum and the opposite LV posterior wall [23, 24, 41].
Electrocardiographic Evaluation
Duration of QRS was assessed from the surface ECG during sinus rhythm (control group), RV pacing (RVP group), and LV pacing (LVP group) and used as a measure for synchrony of electrical ventricular activation.
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) on every parameter. Only if ANOVA showed a significant difference was further analysis with Tukey comparison used to identify statistical differences between the different study groups. A p value less than 0.05 determined statistical significance. Data are presented as mean ± standard deviation. For echocardiographic measures, the interobserver correlation (r value) was assessed using Pearson product-moment correlation.
Results
Study Population
The characteristics of the RVP and LVP groups are presented in Table 1. Unless specified, the etiology of the AV block was not identified. Age and body surface area were not significantly different between the groups (RVP group: 6.6 ± 2.2 years and 0.9 ± 0.2 m2, respectively; LVP group: 6.7 ± 3.3 years and 0.9 ± 0.3 m2; control group: 4.8 ± 3.7 years and 0.7 ± 0.3 m2). The duration of ventricular pacing was not significantly different between the two paced groups (RVP: 3.9 ± 2.1 years vs LVP: 3.8 ± 1.8 years).
Due to the paucity of preimplantation echocardiographic data, no statistical analysis was performed on these data. It seems likely from available preimplantation echocardiograms (3 RVP and 4 LVP) that mechanical asynchrony during AV nodal escape rhythm was irrelevant because SPWMD was 48 ± 33 ms in the RVP group and 23 ± 22 ms in the LVP group. Furthermore, available data suggest no preimplantation difference in shortening fraction between the two groups (RVP: 35.4% ± 6.2% vs LVP: 36.5% ± 3.3%).
Structural Echocardiographic Outcome
Interobserver reproducibility was good for LVEDD (r = 0.96), LVESD (r = 0.95), mean LVEDWT (r = 0.90), and mean LVESWT (r = 0.94). Among the three study groups, LVEDD, LVESD, mean LVEDWT, and mean LVESWT did not differ significantly (Table 2). No significant differences in LV wall thickness between different regions of the heart were observed in any of the study groups (data not shown).
Functional Echocardiographic Outcome
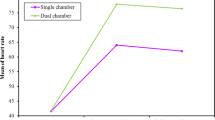
After chronic epicardial pacing, the shortening fraction was significantly lower in the RVP group (21.7% ± 6.0%) than in the LVP group (32.2 ± 5.2%) or the control group (29.3% ± 4.3%, nonsignificant difference between the control and LVP groups, Fig. 2). The end-systolic LV eccentricity index was significantly higher in the RVP group (1.8 ± 0.2) than in the LVP (1.4 ± 0.1) or control (1.4 ± 0.2) group (nonsignificant difference between the LVP and control groups, Fig. 3).
End-systolic left ventricular eccentricity index in the study groups. For legends see Fig. 2
At end-diastole, the LV eccentricity index was not significantly different between the study groups (RVP: 2.7 ± 0.3; LVP: 2.7 ± 0.6; control: 2.7 ± 0.4). The SPWMD was similar in the control (−5 ± 35 ms) and LVP (−16 ± 14 ms) groups, with good interobserver reproducibility (r = 0.97). As compared with the control and LVP groups, the SPWMD in the RVP group (338 ± 20 ms) was significantly different (p < 0.001).
QRS Duration
The duration of QRS was 69 ± 7 ms in the control group, and significantly longer (p < 0.001) in both paced groups (RVP: 157 ± 10 ms vs LVP: 158 ± 22 ms; nonsignificant difference between RVP and LVP).
Discussion
To our knowledge, this is the first study to compare the long-term effects of RV and LV pacing in children. The current study supports the finding of previous studies that chronic RV pacing can be detrimental to LV function [16, 19, 30, 31]. Chronic RV pacing is associated with deleterious LV remodeling [15, 31] and decreased LV function [30] in the young (for a review, see Karpawich [16]) and in adults (for review see Manolis [19]). More importantly, the current study shows that during chronic epicardial LV pacing (mean duration, >3½ years), LV function and structure are maintained at the level of healthy control children. Therefore, the pacing site appears to be an important determinant for cardiac function and structure in children.
Pacing Site and LV Structure
Animal experiments showed asymmetrical hypertrophy during pacing [35], because early-activated regions of the LV had a low workload due to a low-pressure gradient at this early activation phase. Low workload results in hypotrophy, whereas late-activated regions hypertrophy as they are stretched before activation and thus must perform higher myocardial work (local Frank-Starling effect). Remarkably, we did not find differences in regional wall thicknesses during pacing, possibly because the quality of images was not appropriate to distinct regional changes in wall thicknesses.
Relation Between Pacing Site and LV Function
In parallel with the acute decrease in cardiac pump function during RV pacing [14, 36], chronic RV pacing significantly depressed shortening fraction in the current study. Furthermore, the LV eccentricity index was significantly increased after chronic RV pacing. This eccentricity index provides an approximation of fiber stress because fiber stress increases in proportion to the result for the following equation [2, 7]:
from this equation, it can be deduced that at a similar LV pressure, an increase in LV cavity diameter or a decrease in LV wall thickness will result in higher fiber stress. Although neither LV nor aortic pressures were assessed for the children in this study, the increased eccentricity index in RV-paced children may indicate increased fiber stress.
Left ventricular pacing neither increased the eccentricity index nor decreased the shortening fraction after more than 3 years of pacing. Superior hemodynamic performance during LV pacing compared with RV pacing probably is caused by a more favorable balance between interventricular synchrony [13], intraventricular synchrony [36], and the sequence of electrical ventricular activation [26] during LV pacing. The idea that the sequence of activation is an important determinant of cardiac pump function is supported by other studies as well [25, 26].
During RV pacing, a left bundle branch block pattern of activation is created [16, 19, 26, 36]. In the current study, RV-paced children showed significant intraventricular mechanical dyssynchrony, with motion of the interventricular septum toward the LV posterior wall more than 300 ms before the first peak of inward LV posterior wall movement. This motion pattern of the interventricular septum is caused by the pressure developments within each ventricle. If the interventricular septum and the LV free wall are activated after the RV free wall, RV pressure increases before LV pressure is built up. This causes early systolic bulging of the interventricular septum into the LV [18].
Cardiac resynchronization therapy with biventricular pacing increases cardiac function after surgery for patients with congenital heart disease compared with intrinsic activation [13, 42]. Experience with chronic biventricular pacing in children is sparse, but a multicenter study showed promising results with regard to cardiac function after 4 months of resynchronization therapy [11], and biventricular pacing proved effective in the treatment of six children with RV pacing-induced heart failure [20]. In adults with congestive heart failure, chronic LV lateral wall pacing (single-site, short AV delay) can be as effective as biventricular pacing [4, 12, 33]. To our knowledge, the effect of chronic biventricular pacing in children has not been compared with LV lateral wall pacing alone.
The duration of QRS was not significantly different between the two paced groups in our study despite the significantly different hemodynamic and mechanical performance. This is consistent with other studies, in which QRS duration during pacing was not related to cardiac function [25, 36, 42]. It is important to bear in mind that QRS duration reflects total biventricular activation time, whereas intraventricular mechanical synchrony and sequence of electrical ventricular activation [26] probably are more important determinants of LV function [19, 36]. Given the absence of a consistent correlation between cardiac function and QRS duration in acute and chronic pacing studies, we discourage the use of QRS duration as a tool for the selection of an optimal epicardial pacing site in children.
Clinical Application
We apply and advocate the use of LV pacing sites when chronic epicardial pacing is indicated in children. We do so because chronic LV pacing has proved superior to RV pacing in terms of LV function and relative systolic dimensions in the current study, and because in some case reports, RV pacing-induced heart failure was successfully treated with single-site LV pacing [29, 32, 37]. However, once implantation of LV epicardial leads is started, it is of major clinical importance to know which site, apex, or free wall should be preferred. In an acute-pacing study, LV apex pacing increased pump function compared with RV pacing, whereas there was no benefit of LV free wall pacing [36]. Nevertheless, the current study shows that LV lateral wall pacing preserves LV function.
These findings could be explained by subtle differences in LV lateral wall pacing sites. In the aforementioned study [36], the pacing lead was placed at the base of the LV lateral wall, whereas in the current study, the location of the LV pacing lead was halfway between the LV apex and the base of the LV lateral wall (mid lateral wall).
The hypothesis that the exact pacing site at the LV lateral wall influences the effect of LV pacing is supported by the following experiment. In an established animal left bundle branch block (LBBB) model [39, 40], we investigated the hemodynamic improvement of four epicardial LV pacing sites. Pacing was applied to seven dogs with experimental LBBB at four LV pacing sites: apex, apical lateral wall, mid lateral wall, and base of the lateral wall, respectively. At all sites, pacing was performed after the same short AV delay to avoid fusion with intrinsic activation. The averaged maximum rate of LV pressure rise (LVdP/dt|max) from all beats during one ventilation cycle, measured with a catheter tip manometer, was used as a measure of LV function. Compared with LBBB, LVdP/dt|max was significantly increased by pacing at the LV apex (18% ± 11%; p = 0.005), LV apical lateral wall (11% ± 6%; p = 0.001), and LV mid lateral wall (7% ± 6%; p = 0.020), whereas no significant improvement in LV function occurred during pacing at the base of the LV lateral wall (3% ± 10%; p = 0.413).
Although pacing at the LV apex caused the most pronounced improvement in LV function, it is not easy to come within reach of this particular pacing site using established endovenous or minimal surgical techniques. However, pacing at epicardial LV mid lateral wall sites also resulted in improved LV function and is easily accessible through a left lateral thoracotomy. This approach is surgically reliable and provides excellent cosmetic and functional results in children [9].
Study Limitations
Although the number of patients studied in the current series was small, we consider the study groups to be comparable because body surface area, age, and duration of pacing were similar, and none of the patients had structural heart disease.
Because echocardiographic image quality deteriorates during long-term storage [34], we were hampered in our retrospective evaluation, especially in the evaluation of preimplantation data. We therefore could not achieve a longitudinal follow-up evaluation of all the patients and could not perform statistical analysis of echocardiographic preimplantation data. Therefore, the possibility that some of the differences between chronically RV- and LV-paced children were preexisting cannot entirely be excluded.
The main disadvantage of this study is its retrospective design. The positive effects of single-site LV pacing observed in this study, as well as the practical advantages of single-site over multisite ventricular pacing, strongly advocate further investigation on LV pacing in prospective multicenter studies.
Conclusion
Left ventricular function in children is preserved by chronic pacing at the LV lateral wall, whereas chronic RV pacing causes a decrease in shortening fraction and a higher systolic eccentricity index.
References
Andersen HR, Nielsen JC, Thomsen PE et al (1997) Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet 350:1210–1216
Arts T, Bovendeerd PH, Prinzen FW, Reneman RS (1991) Relation between left ventricular cavity pressure and volume and systolic fiber stress and strain in the wall. Biophys J 59:93–102
Blanc JJ, Etienne Y, Gilard M et al (1997) Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation 96:3273–3277
Blanc JJ, Bertault-Valls V, Fatemi M et al (2004) Midterm benefits of left univentricular pacing in patients with congestive heart failure. Circulation 109:1741–1744
Daubeney PE, Blackstone EH, Weintraub RG et al (1999) Relationship of the dimension of cardiac structures to body size: an echocardiographic study in normal infants and children. Cardiol Young 9:402–410
de Cock CC, Giudici MC, Twisk JW (2003) Comparison of the haemodynamic effects of right ventricular outflow-tract pacing with right ventricular apex pacing: a quantitative review. Europace 5:275–278
Delhaas T, Arts T, Bovendeerd PH et al (1993) Subepicardial fiber strain and stress as related to left ventricular pressure and volume. Am J Physiol 264:H1548–H1559
Deshmukh P, Casavant DA, Romanyshyn M, Anderson K (2000) Permanent, direct his-bundle pacing: a novel approach to cardiac pacing in patients with normal his-purkinje activation. Circulation 101:869–877
Dodge-Khatami A, Kadner A, Dave H et al (2005) Left heart atrial and ventricular epicardial pacing through a left lateral thoracotomy in children: a safe approach with excellent functional and cosmetic results. Eur J Cardiothorac Surg 28:541–545
Du Bois D, Du Bois EF (1989) A formula to estimate the approximate surface area if height and weight be known: 1916. Nutrition 5:303–311; (discussion 312–303)
Dubin AM, Janousek J, Rhee E et al (2005) Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J Am Coll Cardiol 46:2277–2283
Etienne Y, Mansourati J, Gilard M et al (1999) Evaluation of left ventricular-based pacing in patients with congestive heart failure and atrial fibrillation. Am J Cardiol 83:1138–1140, A1139
Janousek J, Vojtovic P, Hucin B et al (2001) Resynchronization pacing is a useful adjunct to the management of acute heart failure after surgery for congenital heart defects. Am J Cardiol 88:145–152
Karpawich PP, Mital S (1997) Comparative left ventricular function following atrial, septal, and apical single chamber heart pacing in the young. Pacing Clin Electrophysiol 20:1983–1988
Karpawich PP, Rabah R, Haas JE (1999) Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol 22:1372–1377
Karpawich PP (2004) Chronic right ventricular pacing and cardiac performance: the pediatric perspective. Pacing Clin Electrophysiol 27:844–849
Kim JJ, Friedman RA, Eidem BW et al (2007) Ventricular function and long-term pacing in children with congenital complete atrioventricular block. J Cardiovasc Electrophysiol 18:373–377
Lumens J, Delhaas T, Kirn B, Arts T (2008) Modeling ventricular interaction: a multiscale approach from sarcomere mechanics to cardiovascular system hemodynamics. Pac Symp Biocomput 13:378–389
Manolis AS (2006) The deleterious consequences of right ventricular apical pacing: time to seek alternate site pacing. Pacing Clin Electrophysiol 29:298–315
Moak JP, Hasbani K, Ramwell C et al (2006) Dilated cardiomyopathy following right ventricular pacing for AV block in young patients: resolution after upgrading to biventricular pacing systems. J Cardiovasc Electrophysiol 17:1068–1071
Nelson GS, Berger RD, Fetics BJ et al (2000) Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation 102:3053–3059
Peschar M, de Swart H, Michels KJ et al (2003) Left ventricular septal and apex pacing for optimal pump function in canine hearts. J Am Coll Cardiol 41:1218–1226
Pitzalis MV, Iacoviello M, Romito R et al (2002) Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol 40:1615–1622
Pitzalis MV, Iacoviello M, Romito R et al (2005) Ventricular asynchrony predicts a better outcome in patients with chronic heart failure receiving cardiac resynchronization therapy. J Am Coll Cardiol 45:65–69
Prinzen FW, Van Oosterhout MF, Vanagt WY et al (1998) Optimization of ventricular function by improving the activation sequence during ventricular pacing. Pacing Clin Electrophysiol 21:2256–2260
Prinzen FW, Peschar M (2002) Relation between the pacing induced sequence of activation and left ventricular pump function in animals. Pacing Clin Electrophysiol 25:484–498
Puggioni E, Brignole M, Gammage M et al (2004) Acute comparative effect of right and left ventricular pacing in patients with permanent atrial fibrillation. J Am Coll Cardiol 43:234–238
Sweeney MO, Hellkamp AS, Ellenbogen KA et al (2003) Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 107:2932–2937
Takasugi H, Watanabe K, Ono Y, Echigo S (2005) Improvement of left ventricular function after changing the pacing site in a child with isolated congenital complete atrioventricular block and dilated cardiomyopathy. Pediatr Cardiol 26:87–89
Tantengco MV, Thomas RL, Karpawich PP (2001) Left ventricular dysfunction after long-term right ventricular apical pacing in the young. J Am Coll Cardiol 37:2093–2100
Thambo JB, Bordachar P, Garrigue S et al (2004) Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation 110:3766–3772
Tissot C, Aggoun Y, Rimensberger PC et al (2007) Left ventricular epicardial VVI pacing for a congenital complete heart block with severe myocardial dysfunction: shall epicardial pacing wires be positioned left? Int J Cardiol 116:e7–e9
Touiza A, Etienne Y, Gilard M et al (2001) Long-term left ventricular pacing: assessment and comparison with biventricular pacing in patients with severe congestive heart failure. J Am Coll Cardiol 38:1966–1970
Van Bogart JWC (1996) What can go wrong with magnetic media? Pub Res Q 12:65–77
van Oosterhout MF, Prinzen FW, Arts T et al (1998) Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation 98:588–595
Vanagt WY, Verbeek XA, Delhaas T et al (2004) The left ventricular apex is the optimal site for pediatric pacing. Pacing Clin Electrophysiol 27:837–843
Vanagt WY, Prinzen FW, Delhaas T (2007) Reversal of pacing-induced heart failure by left ventricular apical pacing. N Engl J Med 357:2637–2638
Vatasescu R, Shalganov T, Paprika D et al (2007) Evolution of left ventricular function in paediatric patients with permanent right ventricular pacing for isolated congenital heart block: a medium term follow-up. Europace 9:228–232
Verbeek XA, Vernooy K, Peschar M et al (2003) Intraventricular resynchronization for optimal left ventricular function during pacing in experimental left bundle branch block. J Am Coll Cardiol 42:558–567
Vernooy K, Verbeek XA, Peschar M et al (2005) Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J 26:91–98
Vernooy K, Cornelussen RNM, Verbeek XAAM et al (2007) Cardiac resynchronization therapy cures dyssynchronopathy in canine left bundle-branch block hearts. Eur Heart J 28:2148–2155
Zimmerman FJ, Starr JP, Koenig PR et al (2003) Acute hemodynamic benefit of multisite ventricular pacing after congenital heart surgery. Ann Thorac Surg 75:1775–1780
Acknowledgments
The authors gratefully acknowledge Joost Lumens, MSc, at the Maastricht University for his contribution to the data analysis. They thank St. Jude Medical, Veenendaal, The Netherlands, for financial support of the travel expenses related to this project. Frits Prinzen is consultant to Medtronic Inc. (Minneapolis, MN, USA) and Boston Scientific Corp. (St. Paul, MN, USA).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Geldorp, I.E., Vanagt, W.Y., Bauersfeld, U. et al. Chronic Left Ventricular Pacing Preserves Left Ventricular Function in Children. Pediatr Cardiol 30, 125–132 (2009). https://doi.org/10.1007/s00246-008-9284-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-008-9284-2