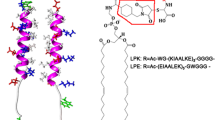
Abstract
We show that the interaction of aromatic amino acids with lipid bilayers can be characterized by conventional 1D \(^1\)H NMR spectroscopy using reference spectra obtained in isopropanol-d8/D\(_{2}\)O solutions. We demonstrate the utility of this method with three different peptides containing tyrosine, tryptophan, or phenylalanine amino acids in the presence of 1,2-dioleoyl-sn-glycero-3-phosphocholine or 1,2-dioleoyl-sn-glycero-3-phosphoserine lipid membranes. In each case, we determine an equivalent isopropanol concentration (EIC) for each hydrogen site of aromatic groups, in essence constructing a map of the chemical environment. These EIC maps provide information on relative affinities of aromatic side chains for either PC or PS bilayers and also inform on amino acid orientation preference when bound to membranes.







Similar content being viewed by others
References
Balestrieri C, Colonna G, Giovane A (1978) Second-derivative spectroscopy of proteins. Eur J Biochem 90(3):433–440
Balestrieri C, Colonna G, Giovane A, Irace G, Servillo L (1980) Second-derivative spectroscopy of proteins: studies on tyrosyl residues. Anal Biochem 106(1):49–54
Chelli R, Gervasio FL, Procacci P, Schettino V (2002) Stacking and t-shape competition in aromatic-aromatic amino acid interactions. J Am Chem Soc 124(21):6133–6143
de Planque MRR, Bonev BB, Demmers JAA, Greathouse DV, II Koeppe RE (2003) Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide-lipid interactions. Biochemistry 42:5341–5348
Esfandiary R, Hunjan JS, Lushington GH, Joshi SB, Middaugh CR (2009) Temperature dependent 2nd derivative absorbance spectroscopy of aromatic amino acids as a probe of protein dynamics. Protein Sci 18:2603–2614
Fernstrom JD, Fernstrom MH (2007) Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr 137(6):1539–1547
Garcia-Borron J, Escribano J, Jimenez M, Iborra J (1982) Quantitative determination of tryptophanyl and tyrosyl residues of proteins by second-derivative fluorescence spectroscopy. Anal Biochem 125(2):227–285
Gelan J, Anteunis M (1972) \(^1\)H 300 MHz NMR spectra of the natural amino acids in neutral-, acid-, and basic aqueous solutions, Laboratory NMR Spectroscopy Organic Chemistry Rijksuniversiteit Gent
Gleason NJ, Vostrikov VV, Greathouse DV, Grant CV, Opella SJ, Koeppe RE (2012) Tyrosine replacing tryptophan as an anchor in gwalp peptides. Biochemistry 51:2044–2053
Heine T, Corminboeuf C, Seifert G (2005) The magnetic shielding functionnction of molcules and pi-electron delocalizaiton. Chem Rev 105:3889–3910
Karadakov PB, Horner KE (2013) Magnetic shielding in and around benzene and cyclobutadiene: a source of information about aromaticity, antiaromaticity, and chemical bonding. J Phys Chem A 117(2):518–523
Killian J, von Heijne G (2000) How proteins adapt to a membrane-water interface. Trends Biochem Sci 25(9):429–434
Levine RL, Federicit MM (1982) Quantitation of aromatic residues in proteins: model compounds for second-derivative spectroscopy. Biochemistry 21:2600–2606
Mach H, Middaugh CR (1994) Simultaneous monitoring of the environment of tryptophan, tyrosine, and phenylalanine residues in proteins by near-ultraviolet second-derivative spectroscopy. Anal Biochem 222:323–331
Moon CP, Fleming KG (2011) Side-chain hydrophobicity scale derived from transmembrane protein folding into lipid bilayers. PNAS 108(25):10,174–10,177
Mozo-Villarías A (2002) Second derivative fluorescence spectroscopy of tryptophan in proteins. J Biochem Biophys Methods 50:163–178
Nyar S, Brahma A, Mukherjee C, Bhattacharyya D (2002) Second derivative fluorescence spectra of indole compounds. J Biochem 131:427–435
Phillips R, Ursell T, Wiggins P, Sens P (2009) Emerging roles for lipids in shaping membrane-protein function. NATURE 459:379–385
Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, Bremer HJ (1999) Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest 103(8):1169–1178
Shulkin BL, Betz AL, Koeppe RA, Agranoff BW (1995) Inhibition of neutral amino acid transport across the human blood-brain barrier by phenylalanine. J Neurochem 64(3):1252–1257
Stevens TJ, Arkin IT (2000) Do more complex organisms have a greater proportion of membrane proteins in their genomes? PROTEINS 39:417–420
Ulrich EL, Akutsu H, Doreleijers JF, Harano Y, Ioannidis YE, Lin J, Livny M, Mading S, Maziuk D, Miller Z, Nakatani E, Schulte CF, Tolmie DE, Wenger RK, Yao H, Markley JL (2008) Biomagresbank. Nucl Acids Res 36:D402–D408
van Beek JD (2007) matNMR: a flexible toolbox for processing, analyzing and visualizing magnetic resonance data in matlab. J Magn Reson 187:19–26
Weizenmann N, Huster D, Scheidt HA (2012) Interaction of local anesthetics with lipid bilayers investigated by \(^1\)H MAS NMR spectroscopy. Biochim et Biophys Acta 1818 1818:3010–3018
White SH (2003) Translocons, thermodynamics, and the folding of membrane proteins. FEBS Lett 555:116–121
Yau WM, Wimley WC, Gawrisch K, White SH (1998) The preference of tryptophan for membrane interfaces. Biochemistry 37:14,713–14,718
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, M.A., Ray, B.D., Wassall, S.R. et al. Equivalent Isopropanol Concentrations of Aromatic Amino Acids Interactions with Lipid Vesicles. J Membrane Biol 248, 695–703 (2015). https://doi.org/10.1007/s00232-015-9781-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-015-9781-1