Abstract
Aim
To investigate whether single-nucleotide polymorphisms (SNPs) in the P450 oxidoreductase (POR) gene were correlated with interindividual variations in cytochrome P450 (CYP) 2B6 activity.
Methods
Thirty-six healthy volunteers who tested CYP2B6 and POR polymorphisms were enrolled in the study. CYP2B6 activity was measured by bupropion hydroxylation with LC/MS/MS. The ratio of hydroxybupropion versus bupropion (AUC_hyd/AUC_bup) in terms of area under the time-concentration curve (AUC) was used to represent the CYP2B6 activity.
Results
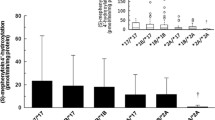
The volunteers carrying CYP2B6*1/*1 showed a significantly higher mean AUC_hyd/ AUC_bup than those CYP2B6*1/*6 and CYP2B6*6/*6 variants (15.66 ± 1.65 vs. 9.25 ± 1.92, P = 0.008 and 15.66 ± 1.65 vs. 8.21 ± 1.74, P = 0.006, respectively). POR rs2868177 (6593 A > G) AA homozygotes showed a significantly lower mean AUC_hyd/ AUC_bup than that of POR rs2868177 AG heterozygotes or GG homozygotes (8.13 ± 1.37 vs. 12.15 ± 2.97, P = 0.005 and 8.13 ± 1.37 vs. 17.59 ± 3.25, P = 0.001, respectively). Moreover, POR rs2868177 AG heterozygotes and GG homozygotes showed a significantly increased mean AUC_hyd/AUC_bup than AA homozygotes in the CYP2B6*1/*1 and CYP2B6*6 carriers (16.40 ± 2.01 vs. 12.40 ± 1.45, P = 0.006 and 10.65 ± 1.47 vs. 6.54 ± 1.25, P = 0.004, respectively). Meanwhile, a strong correlation between the genetic variations (POR rs2868177 and CYP2B6*6) and AUC_hyd/ AUC_bup was found (P = 0.009 and P = 0.001, respectively). There was no significant difference in the mean AUC_hyd/AUC_bup among different POR *28 genotypes (P > 0.05).
Conclusion
POR rs2868177 and CYP2B6*6 variants contribute to the interindividual variability in human CYP2B6 activity, which may affect the disposition and interaction of other CYP2B6 substrate drugs.



Similar content being viewed by others
References
Zanger UM, Klein K, Saussele T, Blievernicht J, Hofmann MH, Schwab M (2007) Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics 8(7):743–759. doi:10.2217/14622416.8.7.743
Wang H, Tompkins LM (2008) CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab 9(7):598–610
Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ (2000) CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos 28(10):1176–1183
Svensson US, Ashton M (1999) Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol 48(4):528–535
Court MH, Duan SX, Hesse LM, Venkatakrishnan K, Greenblatt DJ (2001) Cytochrome P-450 2B6 is responsible for interindividual variability of propofol hydroxylation by human liver microsomes. Anesthesiology 94(1):110–119
Kharasch ED, Hoffer C, Whittington D, Sheffels P (2004) Role of hepatic and intestinal cytochrome P450 3 A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Clin Pharmacol Ther 76(3):250–269. doi:10.1016/j.clpt.2004.05.003
Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM (2000) Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos 28(10):1222–1230
Hou YC, Lai CH (2015) The accompanying changes in brain structure of a remitted depression patient with the bupropion treatment. Clin Psychopharmacol Neurosci 13(3):319–320. doi:10.9758/cpn.2015.13.3.319
Vogeler T, McClain C, Evoy KE (2016) Combination bupropion SR and varenicline for smoking cessation: a systematic review. Am J Drug Alcohol Abuse 42(2):129–139. doi:10.3109/00952990.2015.1117480
Zhang H, Sridar C, Kenaan C, Amunugama H, Ballou DP, Hollenberg PF (2011) Polymorphic variants of cytochrome P450 2B6 (CYP2B6.4-CYP2B6.9) exhibit altered rates of metabolism for bupropion and efavirenz: a charge-reversal mutation in the K139E variant (CYP2B6.8) impairs formation of a functional cytochrome p450-reductase complex. J Pharmacol Exp Ther 338(3):803–809. doi:10.1124/jpet.111.183111
Zanger UM, Klein K (2013) Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front Genet 4:24. doi:10.3389/fgene.2013.00024
Li J, Menard V, Benish RL, Jurevic RJ, Guillemette C, Stoneking M, Zimmerman PA, Mehlotra RK (2012) Worldwide variation in human drug-metabolism enzyme genes CYP2B6 and UGT2B7: implications for HIV/AIDS treatment. Pharmacogenomics 13(5):555–570. doi:10.2217/pgs.11.160
Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, Eichelbaum M, Schwab M, Zanger UM (2001) Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics 11(5):399–415
Loboz KK, Gross AS, Williams KM, Liauw WS, Day RO, Blievernicht JK, Zanger UM, McLachlan AJ (2006) Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin Pharmacol Ther 80(1):75–84. doi:10.1016/j.clpt.2006.03.010
Benowitz NL, Zhu AZ, Tyndale RF, Dempsey D, Jacob P 3rd (2013) Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet Genomics 23(3):135–141. doi:10.1097/FPC.0b013e32835d9ab0
Gigon PL, Gram TE, Gillette JR (1969) Studies on the rate of reduction of hepatic microsomal cytochrome P-450 by reduced nicotinamide adenine dinucleotide phosphate: effect of drug substrates. Mol Pharmacol 5(2):109–122
Agrawal V, Choi JH, Giacomini KM, Miller WL (2010) Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase. Pharmacogenet Genomics 20(10):611–618. doi:10.1097/FPC.0b013e32833e0cb5
Henderson CJ, Otto DM, Carrie D, Magnuson MA, McLaren AW, Rosewell I, Wolf CR (2003) Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J Biol Chem 278(15):13480–13486. doi:10.1074/jbc.M212087200
Otto DM, Henderson CJ, Carrie D, Davey M, Gundersen TE, Blomhoff R, Adams RH, Tickle C, Wolf CR (2003) Identification of novel roles of the cytochrome p450 system in early embryogenesis: effects on vasculogenesis and retinoic acid homeostasis. Mol Cell Biol 23(17):6103–6116
Huang N, Agrawal V, Giacomini KM, Miller WL (2008) Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc Natl Acad Sci U S A 105(5):1733–1738. doi:10.1073/pnas.0711621105
Zhang X, Li L, Ding X, Kaminsky LS (2011) Identification of cytochrome P450 oxidoreductase gene variants that are significantly associated with the interindividual variations in warfarin maintenance dose. Drug Metab Dispos 39(8):1433–1439. doi:10.1124/dmd.111.038836
de Jonge H, Metalidis C, Naesens M, Lambrechts D, Kuypers DR (2011) The P450 oxidoreductase *28 SNP is associated with low initial tacrolimus exposure and increased dose requirements in CYP3A5-expressing renal recipients. Pharmacogenomics 12(9):1281–1291. doi:10.2217/pgs.11.77
Chen X, Pan LQ, Naranmandura H, Zeng S, Chen SQ (2012) Influence of various polymorphic variants of cytochrome P450 oxidoreductase (POR) on drug metabolic activity of CYP3A4 and CYP2B6. PLoS One 7(6):e38495. doi:10.1371/journal.pone.0038495
Gijsen VM, van Schaik RH, Soldin OP, Soldin SJ, Nulman I, Koren G, de Wildt SN (2014) P450 oxidoreductase *28 (POR*28) and tacrolimus disposition in pediatric kidney transplant recipients—a pilot study. Ther Drug Monit 36(2):152–158. doi:10.1097/FTD.0b013e3182a3f282
Drogari E, Ragia G, Mollaki V, Elens L, Van Schaik RH, Manolopoulos VG (2014) POR*28 SNP is associated with lipid response to atorvastatin in children and adolescents with familial hypercholesterolemia. Pharmacogenomics 15(16):1963–1972. doi:10.2217/pgs.14.138
Gao L, He Y, Tang J, Yin J, Huang Z, Liu F, Ouyang D, Chen X, Zhang W, Liu Z, Zhou H (2013) Genetic variants of pregnane X receptor (PXR) and CYP2B6 affect the induction of bupropion hydroxylation by sodium ferulate. PLoS One 8(6):e62489. doi:10.1371/journal.pone.0062489
Yang G, Fu Z, Chen X, Yuan H, Yang H, Huang Y, Ouyang D, Tan Z, Tan H, Huang Z, Zhou H (2011) Effects of the CYP oxidoreductase Ala503Val polymorphism on CYP3A activity in vivo: a randomized, open-label, crossover study in healthy Chinese men. Clin Ther 33(12):2060–2070. doi:10.1016/j.clinthera.2011.11.004
Lv J, Hu L, Zhuo W, Zhang C, Zhou H, Fan L (2016) Effects of the selected cytochrome P450 oxidoreductase genetic polymorphisms on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Pharmacogenet Genomics 26(2):80–87. doi:10.1097/FPC.0000000000000190
Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, Tyndale RF (2007) CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol Psychiatry 62(6):635–641. doi:10.1016/j.biopsych.2006.10.005
Tan SL, Li Z, Zhang W, Song GB, Liu LM, Peng J, Liu ZQ, Fan L, Meng XG, Wang LS, Chen Y, Zhou XM, Zhou HH (2013) Cytochrome P450 oxidoreductase genetic polymorphisms A503 V and rs2868177 do not significantly affect warfarin stable dosage in Han-Chinese patients with mechanical heart valve replacement. Eur J Clin Pharmacol 69(10):1769–1775. doi:10.1007/s00228-013-1544-2
Chung JY, Cho JY, Lim HS, Kim JR, KS Y, Lim KS, Shin SG, Jang IJ (2011) Effects of pregnane X receptor (NR1I2) and CYP2B6 genetic polymorphisms on the induction of bupropion hydroxylation by rifampin. Drug Metab Dispos 39(1):92–97. doi:10.1124/dmd.110.035246
Haroun F, Al-Shaar L, Habib RH, El-Saghir N, Tfayli A, Bazarbachi A, Salem Z, Shamseddine A, Taher A, Cascorbi I, Zgheib NK (2015) Effects of CYP2B6 genetic polymorphisms in patients receiving cyclophosphamide combination chemotherapy for breast cancer. Cancer Chemother Pharmacol 75(1):207–214. doi:10.1007/s00280-014-2632-4
Lim YP, Cheng CH, Chen WC, Chang SY, Hung DZ, Chen JJ, Wan L, Ma WC, Lin YH, Chen CY, Yokoi T, Nakajima M, Chen CJ (2015) Allyl isothiocyanate (AITC) inhibits pregnane X receptor (PXR) and constitutive androstane receptor (CAR) activation and protects against acetaminophen- and amiodarone-induced cytotoxicity. Arch Toxicol 89(1):57–72. doi:10.1007/s00204-014-1230-x
Thorn CF, Lamba JK, Lamba V, Klein TE, Altman RB (2010) PharmGKB summary: very important pharmacogene information for CYP2B6. Pharmacogenet Genomics 20(8):520–523
Lunde I, Bremer S, Midtvedt K, Mohebi B, Dahl M, Bergan S, Asberg A, Christensen H (2014) The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients. Eur J Clin Pharmacol 70(6):685–693. doi:10.1007/s00228-014-1656-3
Elens L, Nieuweboer AJ, Clarke SJ, Charles KA, de Graan AJ, Haufroid V, van Gelder T, Mathijssen RH, van Schaik RH (2013) Impact of POR*28 on the clinical pharmacokinetics of CYP3A phenotyping probes midazolam and erythromycin. Pharmacogenet Genomics 23(3):148–155. doi:10.1097/FPC.0b013e32835dc113
Sandee D, Morrissey K, Agrawal V, Tam HK, Kramer MA, Tracy TS, Giacomini KM, Miller WL (2010) Effects of genetic variants of human P450 oxidoreductase on catalysis by CYP2D6 in vitro. Pharmacogenet Genomics 20(11):677–686. doi:10.1097/FPC.0b013e32833f4f9b
Gomes AM, Winter S, Klein K, Turpeinen M, Schaeffeler E, Schwab M, Zanger UM (2009) Pharmacogenomics of human liver cytochrome P450 oxidoreductase: multifactorial analysis and impact on microsomal drug oxidation. Pharmacogenomics 10(4):579–599. doi:10.2217/pgs.09.7
O'Leary KA, Li HC, Ram PA, McQuiddy P, Waxman DJ, Kasper CB (1997) Thyroid regulation of NADPH:cytochrome P450 oxidoreductase: identification of a thyroid-responsive element in the 5'-flank of the oxidoreductase gene. Mol Pharmacol 52(1):46–53
Tee MK, Huang N, Damm I, Miller WL (2011) Transcriptional regulation of the human P450 oxidoreductase gene: hormonal regulation and influence of promoter polymorphisms. Mol Endocrinol 25(5):715–731. doi:10.1210/me.2010-0236
Lamba V, Lamba J, Yasuda K, Strom S, Davila J, Hancock ML, Fackenthal JD, Rogan PK, Ring B, Wrighton SA, Schuetz EG (2003) Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J Pharmacol Exp Ther 307(3):906–922. doi:10.1124/jpet.103.054866
Croom EL, Stevens JC, Hines RN, Wallace AD, Hodgson E (2009) Human hepatic CYP2B6 developmental expression: the impact of age and genotype. Biochem Pharmacol 78(2):184–190. doi:10.1016/j.bcp.2009.03.029
Hart SN, Wang S, Nakamoto K, Wesselman C, Li Y, Zhong XB (2008) Genetic polymorphisms in cytochrome P450 oxidoreductase influence microsomal P450-catalyzed drug metabolism. Pharmacogenet Genomics 18(1):11–24. doi:10.1097/FPC.0b013e3282f2f121
Dunner DL, Zisook S, Billow AA, Batey SR, Johnston JA, Ascher JA (1998) A prospective safety surveillance study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 59(7):366–373
Johnston JA, Fiedler-Kelly J, Glover ED, Sachs DP, Grasela TH, DeVeaugh-Geiss J (2001) Relationship between drug exposure and the efficacy and safety of bupropion sustained release for smoking cessation. Nicotine Tob Res 3(2):131–140. doi:10.1080/14622200110042852
Acknowledgments
This work was supported by the Grant from Science and Technology Agency of Hunan Province (2014SK3011), the Scientific Research Funds of Pharmaceutical Association of Hunan Province (xy2015009), Key Projects of Scientific Research Funds in Central Hospital of Changsha in 2016 (CHCS201602), and the Grant from Special Major Science and Technology during the Twelfth Five-Year Plan of People’s Republic of China (2013ZX10005004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study protocol was approved by the ethics committee of Central South University, Changsha, Hunan, P. R. China (approved number: CTXY-110,003) and registered in the Chinese Clinical Trial Registry (registration number: ChiCTR-TRC-11,001,285; Clinical Trial Registry website: http://www.chictr.org.cn /).
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Registration number: ChiCTR-TRC-11,001,285; Scientific title: The effect of ferulic acid on metabolites of hydroxy bupropion by CYP2B6 and POR gene; web site: http://www.chictr.org.cn/showproj.aspx?proj=8254.
Gao LC and Liu FQ contributed equally to the work.
Electronic supplementary material
Table S1
(DOC 465 kb)
Rights and permissions
About this article
Cite this article
Gao, Lc., Liu, Fq., Yang, L. et al. The P450 oxidoreductase (POR) rs2868177 and cytochrome P450 (CYP) 2B6*6 polymorphisms contribute to the interindividual variability in human CYP2B6 activity. Eur J Clin Pharmacol 72, 1205–1213 (2016). https://doi.org/10.1007/s00228-016-2095-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2095-0