Abstract
Purpose
Oncological patients are at increasing risk of QT prolongation, a risk factor for ventricular arrhythmia. We assessed impact and risk factors for corrected QT (QTc) prolongation during multiple-cycle chemotherapy.
Methods
We enrolled 100 outpatients initiating chemotherapy in a university center specializing in female cancer. Clinical, drug, laboratory, and 12-lead ECG data collection at baseline and at each chemotherapy cycle was performed.
Results
Enrolled patients were followed for 992 chemotherapy cycles (median 7; interquartile range 6–13); 2438 ECGs were recorded (20; 18–31) 36.8 % pre-therapy, 36.8 % following chemotherapy, and 22.5 % 7–10 days after chemotherapy. Maximum QTc (Max-QTc) was recorded after 4 chemotherapy administrations in >50 % of the entire cohort and also within every subset of patients with prolonged QTc (57 % 471–480 ms; 54 % 481–500 ms; 66 % >500 ms). No cumulative effect on QTc was shown. QTc prolongation was comparable among the various protocols. Prophylactic/supportive drugs were not associated with additional QTc prolongation. Variables independently associated with QTc prolongation >470 ms were age (OR 1.056 95 % CI 1.006–1.108, p = 0.028) and the baseline-first chemotherapy averaged QTc (BC-QTc) (OR 1.092 95 % CI 1.051–1.136), a novel parameter devised for this study. Only BC-QTc maintained significance for QTc >480 ms. BC-QTc >435 ms identified 100 % of patients with Max-QTc >500 ms, 96 % with Max-QTc 481–500 ms, and 66 % with Max-QTc 471–480 ms. Only 29 % of patients with Max-QTc ≤470 ms presented a BC-QTc >435 ms.
Conclusions
Our results confirm the high prevalence of QTc prolongation after chemotherapy. Most of the patients reached Max-QTc after several cycles. BC-QTc may help in stratifying arrhythmic risk in real-world clinical practice.




Similar content being viewed by others
References
Boriani G, Valzania C, Diemberger I, Biffi M, Martignani C, Bertini M, Ziacchi M, Domenichini G, Saporito D, Rapezzi C, Branzi A (2007) Potential of non-antiarrhythmic drugs to provide an innovative upstream approach to the pharmacological prevention of sudden cardiac death. Expert Opin Investig Drugs 16:605–623
Curigliano G, Spitaleri G, Fingert HJ, de Braud F, Sessa C, Loh E, Cipolla C, De Pas T, Goldhirsch A, Shah R (2008) Drug-induced QTc interval prolongation: a proposal towards an efficient and safe anticancer drug development. Eur J Cancer 44:494–500
van Noord C, Eijgelsheim M, Stricker BH (2010) Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol 70:16–23
Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, Volkers C, Haaksma J, de Vries EG, Sleijfer DT, van der Graaf WT (2001) Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol 19:2746–2753
Suter TM, Ewer MS (2013) Cancer drugs and the heart: importance and management. Eur Heart J 34:1102–1111
Strevel EL, Ing DJ, Siu LL (2007) Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 25:3362–3371
Kitagawa K, Kawada K, Morita S, Inada M, Mitsuma A, Sawaki M, Iino S, Inden Y, Murohara T, Imai T, Ando Y (2012) Prospective evaluation of corrected QT intervals and arrhythmias after exposure to epirubicin, cyclophosphamide, and 5-fluorouracil in women with breast cancer. Ann Oncol 23:743–747
Koca D, Salman T, Unek IT, Oztop I, Ellidokuz H, Eren M, Yilmaz U (2011) Clinical and electrocardiography changes in patients treated with capecitabine. Chemotherapy 57:381–387
Tanriverdi O, Meydan N, Barutca S (2012) Long-term effect of trastuzumab on QT dispersion in adjuvant treatment for patients with Her2 receptor positive breast cancer: a pilot study. Med Oncol 29:3265–3271
Lenihan DJ, Kowey PR (2013) Overview and management of cardiac adverse events associated with tyrosine kinase inhibitors. Oncologist 18:900–908
Haigney MC, Zareba W, Nasir JM, McNitt S, McAdams D, Gentlesk PJ, Goldstein RE, Moss AJ, Investigators MI (2009) Gender differences and risk of ventricular tachycardia or ventricular fibrillation. Heart Rhythm 6:180–186
Malik M, Hnatkova K, Schmidt A, Smetana P (2008) Accurately measured and properly heart-rate corrected QTc intervals show little daytime variability. Heart Rhythm 5:1424–1431
Goldenberg I, Moss AJ, Zareba W (2006) QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol 17:333–336
Moss AJ (1993) Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol 72:23B–25B
Bonate PL, Russell T (1999) Assessment of QTc prolongation for non-cardiac-related drugs from a drug development perspective. J Clin Pharmacol 39:349–358
Pratt CM, Ruberg S, Morganroth J, McNutt B, Woodward J, Harris S, Ruskin J, Moye L (1996) Dose-response relation between terfenadine (Seldane) and the QTc interval on the scalar electrocardiogram: distinguishing a drug effect from spontaneous variability. Am Heart J 131:472–480
Kietpeerakool C, Tiyayon J, Suprasert P, Kanjanavanit R, Srisomboon J (2006) Benefit of electrocardiography during front-line combination paclitaxel and carboplatin chemotherapy for epithelial ovarian cancer. J Med Assoc Thai 89:1805–1810
Sarubbi B, Orditura M, Ducceschi V, De Vita F, Santangelo L, Ciaramella F, Catalano G, Iacono A (1997) Ventricular repolarization time indexes following anthracycline treatment. Heart Vessels 12:262–266
Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB (2009) Introducing a new entity: chemotherapy-induced arrhythmia. Europace 11:1579–1586
Smith LH (2012) Torsade de pointes, prolonged QT intervals, and patients with cancer. Clin J Oncol Nurs 16:125–128
Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S (2003) Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 82:282–290
Acknowledgments
This work was supported by the European Community’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement No. 241679—the ARITMO project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the other partners of the ARITMO project.
STROBE statement
The study was conducted and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations.
Conflict of interest
The authors have declared no conflicts of interest.
Author contributions
Igor Diemberger had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Conception and design: Igor Diemberger, Giuseppe Boriani
Financial support: Giuseppe Boriani
Administrative support: Giuseppe Boriani, Claudio Zamagni
Creation of tools for collecting and storing data: Giulia Massaro
Collection and assembly of data: Giulia Massaro, Marta Cubelli, Daniela Rubino, Sara Quercia, Cristian Martignani, Matteo Ziacchi, Mauro Biffi, Alessandra Bernardi, and Nicoletta Cacciari.
Statistical analysis: Igor Diemberger
Data interpretation: Igor Diemberger, Giuseppe Boriani, and Claudio Zamagni
Manuscript draft: Igor Diemberger, Giulia Massaro
Manuscript revision: All authors
Final approval of manuscript: All authors
Role of Sponsor: The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement No. 241679—the ARITMO project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the other partners of the ARITMO project. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
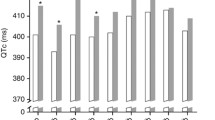
Box plot of QTc values among the entire cohort during the first 6 cycles considering the type of ECG. Legend: Bas = basal ECG, Ch = chemotherapy ECG, Co = control ECG, Pre = pre-therapy ECG. Please note Pre-1 is more correctly reported as Bas. * = p < 0.001 with respect to the previous (pre-therapy) and subsequent ECG (control ECG). (GIF 68 kb)
Figure S2
Scatter plot distribution of maximum QTc over the cycles of chemotherapy (each including: pre-therapy, chemotherapy and control ECG; x-axis) for each patient in accordance with the type of ECG (ECG group). Maximum QTc: normal <450 ms, borderline 450–470 ms, slightly prolongation 471–480 ms, moderate prolongation 481–500 ms, severe prolongation >500 ms. For each category of QTc prolongation the number of included patients is reported. (GIF 37 kb)
Figure S3
Box plot of QTc values of chemotherapy ECGs in accordance with the chemotherapy protocol group (see Table 3). Legend: G = group. Kruskal-Wallis test p < 0.001 for the 6 groups. Bonferroni corrected Mann–Whitney U test p < 0.05 for all the following comparisons: G6 vs. G1-5; G4 vs. G1-3,G5. (GIF 32 kb)
Table S1
(DOC 23 kb)
Rights and permissions
About this article
Cite this article
Diemberger, I., Massaro, G., Cubelli, M. et al. Repolarization effects of multiple-cycle chemotherapy and predictors of QTc prolongation: a prospective female cohort study on >2000 ECGs. Eur J Clin Pharmacol 71, 1001–1009 (2015). https://doi.org/10.1007/s00228-015-1874-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1874-3