Abstract
Purpose
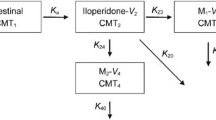
This study evaluated the effects of cytochrome P450 (CYP) 2D6 polymorphisms on the pharmacokinetics of controlled-release paroxetine in healthy Chinese subjects and used paroxetine as a tool drug to compare the performance of traditional phenotype and activity score systems.
Methods
Pharmacokinetic data were evaluated in 24 subjects who received a single oral dose of 25 mg controlled-release paroxetine. Plasma paroxetine concentrations were measured by LC-MS/MS. CYP2D6 genotypes were tested by PCR and direct DNA sequencing. Subjects were classified by two systems of phenotype prediction. In the traditional phenotype system, subjects were classified as extensive metabolizers or intermediate metabolizers; in the activity score system, subjects were divided into four activity groups. Analysis of variance testing was applied to estimate the effects of CYP2D6 polymorphisms on the pharmacokinetics of paroxetine.
Results
With the traditional phenotype system, significant differences were observed in the following pharmacokinetic parameters of paroxetine: t 1/2, C max, AUC0–t, AUC0–inf, Vz/F, and CL/F (all P < 0.05). The AUC or exposure of paroxetine was about 3.5-fold higher in the intermediate metabolizer group than in the extensive metabolizer group. With the activity score system, significant differences were observed in the t 1/2, C max, AUC0–t, AUC0–inf, Vz/F, and CL/F among the four different activity score groups (all P < 0.05). We found that the AUC of paroxetine decreased by around one half as the activity score increased by 0.5.
Conclusion
The pharmacokinetics of controlled-release paroxetine after a single administration was affected by CYP2D6 polymorphisms. Both the traditional phenotype and the activity score systems performed well and distinguished subjects with different drug exposures. The activity score system provided a more detailed classification for the subjects.


Similar content being viewed by others
References
Gibiino S, Serretti A (2012) Paroxetine for the treatment of depression: a critical update. Expert Opin Pharmacother 13(3):421–431
Gilles M, Deuschle M, Kellner S et al (2005) Paroxetine serum concentrations in depressed patients and response to treatment. Pharmacopsychiatry 38(3):118–121
Yasui-Furukori N, Nakagami T, Kaneda A et al (2011) Inverse correlation between clinical response to paroxetine and plasma drug concentration in patients with major depressive disorders. Hum Psychopharmacol 26(8):602–608
Kaye CM, Haddock RE, Langley PF et al (1989) A review of the metabolism and pharmacokinetics of paroxetine in man. Acta Psychiatr Scand Suppl 350:60–75
Jornil J, Jensen KG, Larsen F, Linnet K (2010) Identification of cytochrome P450 isoforms involved in the metabolism of paroxetine and estimation of their importance for human paroxetine metabolism using a population-based simulator. Drug Metab Dispos 38(3):376–385
Ten LK, Bertilsson L (2012) Pharmacogenomics of CYP2D6: molecular genetics, interethnic differences and clinical importance. Drug Metab Pharmacokinet 27(1):55–67
Hicks JK, Swen JJ, Thorn CF, Sangkuhl K, Kharasch ED, Ellingrod VL, Skaar TC, Müller DJ, Gaedigk A, Stingl JC (2013) Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther 93(5):402–408
Gaedigk A (2013) Complexities of CYP2D6 gene analysis and interpretation. Int Rev Psychiatry 25(5):534–553
Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS (2008) The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 83(2):234–242
Saruwatari J (2014) Nakashima H1, Tsuchimine S2, Nishimura M1, Ogusu N1, Yasui-Furukori N2. Possible impact of the CYP2D6*10 polymorphism on the nonlinear pharmacokinetic parameter estimates of paroxetine in Japanese patients with major depressive disorders. Pharmgenomics Pers Med 28(7):121–127
Ververs FF, Voorbij HA, Zwarts P, Belitser SV, Egberts TC, Visser GH, Schobben AF (2009) Effect of cytochrome P450 2D6 genotype on maternal paroxetine plasma concentrations during pregnancy. Clin Pharmacokinet 48(10):677–683
Gex-Fabry M, Eap CB, Oneda B, Gervasoni N, Aubry JM, Bondolfi G, Bertschy G (2008) CYP2D6 and ABCB1 genetic variability: influence on paroxetine plasma level and therapeutic response. Ther Drug Monit 30(4):474–482
Lim KS, Cho JY, Jang IJ, Kim BH, Kim J, Jeon JY, Tae YM, Yi S, Eum S, Shin SG, Yu KS (2008) Pharmacokinetic interaction of flecainide and paroxetine in relation to the CYP2D6*10 allele in healthy Korean subjects. Br J Clin Pharmacol 66(5):660–666
Qian JC, Xu XM, Hu GX, Dai DP, Xu RA, Hu LM, Li FH, Zhang XH, Yang JF, Cai JP (2013) Genetic variations of human CYP2D6 in the Chinese Han population. Pharmacogenomics 14(14):1731–1743
Yoo HD, Cho HY, Lee SN, Yoon H, Lee YB (2012) Population pharmacokinetic analysis of risperidone and 9-hydroxyrisperidone with genetic polymorphisms of CYP2D6 and ABCB1. J Pharmacokinet Pharmacodyn 39(4):329–341
Lee SJ, Lee SS, Jung HJ, Kim HS, Park SJ, Yeo CW, Shin JG (2009) Discovery of novel functional variants and extensive evaluation of CYP2D6 genetic polymorphisms in Koreans. Drug Metab Dispos 37(7):1464–1470
van Nuijs AL, Tarcomnicu I, Simons W, Bervoets L, Blust R, Jorens PG, Neels H, Covaci A (2010) Optimization and validation of a hydrophilic interaction liquid chromatography-tandem mass spectrometry method for the determination of 13 top-prescribed pharmaceuticals in influent wastewater. Anal Bioanal Chem 398(5):2211–2222
Gómez MJ, Petrović M, Fernández-Alba AR, Barceló D (2006) Determination of pharmaceuticals of various therapeutic classes by solid-phase extraction and liquid chromatography-tandem mass spectrometry analysis in hospital effluent wastewaters. J Chromatogr A 1114(2):224–233
Stingl JC, Brockmöller J, Viviani R (2013) Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry 18(3):273–287
Lim JS, Chen XA, Singh O, Yap YS, Ng RC, Wong NS, Wong M, Lee EJ, Chowbay B (2011) Impact of CYP2D6, CYP3A5, CYP2C9 and CYP2C19 polymorphisms on tamoxifen pharmacokinetics in Asian breast cancer patients. Br J Clin Pharmacol 71(5):737–750
Nishida Y, Fukuda T, Yamamoto I, Azuma J (2000) CYP2D6 genotypes in a Japanese population: low frequencies of CYP2D6 gene duplication but high frequency of CYP2D6*10. Pharmacogenetics 10(6):567–570
Zuo LJ, Guo T, Xia DY, Jia LH (2012) Allele and genotype frequencies of CYP3A4, CYP2C19, and CYP2D6 in Han, Uighur, Hui, and Mongolian Chinese populations. Genet Test Mol Biomarkers 16(2):102–108
Ji L, Pan S, Marti-Jaun J, Hänseler E, Rentsch K, Hersberger M (2002) Single-step assays to analyze CYP2D6 gene polymorphisms in Asians: allele frequencies and a novel *14B allele in mainland Chinese. Clin Chem 48(7):983–988
Sindrup SH, Brøsen K, Gram LF et al (1992) The relationship between paroxetine and the sparteine oxidation polymorphism. Clin Pharmacol Ther 51(3):278–287
Acknowledgments
The work was supported by a grant from the National Program on Key Research Project of New Drug Innovation (No. 2012ZX09303006-002). All authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, R., Wang, H., Shi, J. et al. Cytochrome P450 2D6 genotype affects the pharmacokinetics of controlled-release paroxetine in healthy Chinese subjects: comparison of traditional phenotype and activity score systems. Eur J Clin Pharmacol 71, 835–841 (2015). https://doi.org/10.1007/s00228-015-1855-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1855-6