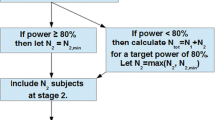
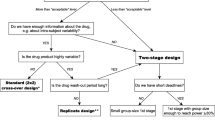
Abstract
Purpose
The aim of this study is to assess the current status of non-fixed sample size designs in bioequivalence trials with a focus on two-stage adaptive approaches.
Methods
We searched PubMed and Google Scholar from inception to October 2014. Regulatory guidelines were obtained from the public domain. Different methods were compared by Monte Carlo simulations for their impact on the patient’s and producer’s risks.
Results
Add-on designs, group sequential designs and adaptive two-stage sequential designs are currently accepted to demonstrate bioequivalence in various regulations. All three approaches may inflate the patient’s risk if applied inconsiderately. Direct transfer of methods developed for superiority testing to bioequivalence is not warranted. Published two-stage frameworks maintain the type I error and generally the desired power. Adaptation based on the observed T/R ratio observed in the first stage should be applied with caution. Monte Carlo simulations are an efficient tool to explore the operating characteristics of methods.
Conclusions
Validated two-stage frameworks can be applied without requiring the sponsor to perform own simulations—which could further improve power based on additional assumptions. Two-stage designs are both ethical and economical alternatives to fixed sample designs.





Similar content being viewed by others
References
Food and Drug Administration, Center for Drug Evaluation and Research (2003) Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. Revision 1. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070124.pdf Accessed 10 October 2014
European Medicines Agency, Committee for Medicinal Products for Human Use (2010) Guideline on the investigation of bioequivalence. CPMP/EWP/QWP/1401/98 Rev. 1. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf Accessed 10 October 2014
Schuirmann DJ(1987) A comparison of the Two One-Sided Test Procedure and the Power Approach for assessing equivalence of average bioavailability. J Pharmacokin Biopharm 15:657–660
McGilveray IJ, Midha KK, Skelly JP, Dighe S, Doluisio JT, French IW, Karim A, Burford R (1990) Consensus report from “Bio International ’89”: issues in the evaluation of bioavailability data. J Pharm Sci 79:945–946. doi:10.1002/jps.2600791022
Melander H (1992) Problems and possibilities with the add-on subject design. In: Midha KK, Blume HH (eds) Bio-International. Bioavailability, bioequivalence and pharmacokinetics. medpharm, Stuttgart, pp 85–90
Commission of the European Communities (1991) CPMP guideline: investigation of bioavailability and bioequivalence. 111/54/89_EN. http://www.clindesc.com/Guidelines_online/3 Clinical/3.1 General/3_1_2.pdf Accessed 10 October 2014
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (1998) ICH harmonised tripartite guideline: statistical principles for clinical trials (E9) http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf Accessed 10 October 2014
Australian Department of Health, Therapeutic Goods Administration (2011) Guideline on the investigation of bioequivalence
Health Canada, Therapeutic Products Directorate (2012) Guidance document: conduct and analysis of comparative bioavailability studies. http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/prodpharma/applic-demande/guide-ld/bio/gd_cbs_ebc_ld-eng.pdf Accessed 10 October 2014
Food and Drug Administration, Office of Generic Drugs (2012) Draft guidance on loteprednol etabonate; tobramycin. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM281453.pdf Accessed 10 October 2014
Food and Drug Administration, Center for Drug Evaluation and Research (2013) Draft Guidance for Industry: bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM377465.pdf Accessed 22 October 2014
Mironov AN, Kukes VG, Petrov VI, Kuznetsov AL, Goryachev DV, Niyazov RR, Prokofiev AB, Nedogoda SV, Frolov MY, Shnaider A (2013) Investigation of bioequivalence of generic medicinal products. In: Mironov AN (ed) Guidelines for clinical trials of drugs. Part 1. Grif and K, Moscow, pp 175–217
Food and Drug Administration, Center for Veterinary Medicine (2014) Draft guidance for industry: bioequivalence: blood level bioequivalence study. VICH GL52. http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM415697.pdf Accessed 06 October 2014
Food and Drug Administration, Center for Veterinary Medicine (2014) Supplemental examples for illustrating statistical concepts described in the VICH in vivo bioequivalence draft guidance GL52. http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM415701.pdf Accessed 06 October 2014
Executive Board of the Health Ministers’ Council for GCC States (2014) The GCC guidelines for bioequivalence. Version 2.3. http://www.sfda.gov.sa/en/drug/drug_reg/Regulations/The%20GCC%20Guidelines%20for%20Bioequivalence%20version%202.3%20GCC%20201.pdf Accessed 29 October 2014
SwissMedic (2014) AW-administrative ordinance: instruction authorisation of human medicines with known active pharmaceutical ingredients, 25 August 2014. https://www.swissmedic.ch/ueber/00134/00519/index.html?lang=en&download=NHzLpZeg7t,lnp6I0NTU042l2Z6ln1ad1IZn4Z2qZpnO2Yuq2Z6gpJCDdX19fmym162epYbg2c_JjKbNoKSn6A-- Accessed 29 October 2014
European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products (2002) Points to consider on multiplicity issues in clinical trials. CPMP/EWP/908/99. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003640.pdf Accessed 10 October 2014
European Medicines Agency, Committee for Medicinal Products for Human Use (2012) Draft concept paper on the need for a guideline on multiplicity issues in clinical trials. EMA/286914/2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/05/WC500127901.pdf Accessed 10 October 2014
Food and Drug Administration, Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research (2010) Draft guidance for industry: adaptive design clinical trials for drugs and biologics. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM201790.pdf Accessed 2 November 2014
European Medicines Agency, Committee for Human Medicinal Products (2007) Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design. CHMP/EWP/2459/02. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003616.pdf Accessed 2 November 2014
Schwartz TA, Denne JS (2003) Common threads between sample size recalculation and group sequential procedures. Pharm Stat 2:263–271. doi:10.1002/pst.068
Dragalin V (2006) Adaptive designs: terminology and classification. Drug Info J 40:425–435
Chow S-C, Chang M (2012) Adaptive design methods in clinical trials, 2nd edn. Chapman & Hall/CRC, Boca Raton
Chin R (2012) Adaptive and flexible clinical trials. Chapman & Hall/CRC, Boca Raton
BEBAC (2014) Guidelines & Guidance Documents. http://bebac.at/Guidelines.htm Accessed 29 October 2014
Labes D (2014) Power2Stage: power and sample size distribution of 2-stage BE studies via simulations. R package version 0.1-5. http://cran.r-project.org/web/packages/Power2Stage/ Accessed 11 October 2014
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Health Canada, Therapeutic Products Directorate (1992) Guidance for industry: conduct and analysis of bioavailability and bioequivalence studies—Part A: oral dosage formulations used for systemic effects
National Institute of Health Science, Division of Drugs (1997) Guideline for bioequivalence studies of generic products. http://www.nihs.go.jp/drug/be-guide(e)/Generic/be97E.pdf Accessed 27 October 2014
New Zealand Medicines and Medical Devices Safety Authority (2001) New Zealand Regulatory Guidelines for Medicines. vol 1, 5th edn
Patterson SD, Zariffa NM-D, Montague TH, Howland K (2001) Non-traditional study designs to demonstrate average bioequivalence for highly variable drug products. Eur J Clin Pharmacol 57:663–670. doi:10.1007/s002280100371
Van Peer A (2010) Variability and impact on design of bioequivalence studies. Basic Clin Pharmacol Toxicol 106:146–153. doi:10.1111/j.1742-7843.2009.00485.x
Administración Nacional de Medicamentos, Alimentos y Tecnología Médica (2006) Disposición ANMAT N° 5040/2006 (con la modificación de la Disp. ANMAT N° 1746/2007) http://www.anmat.gov.ar/webanmat/Legislacion/Medicamentos/Disposicion_ANMAT_5040-2006.pdf Accessed 29 October 2014
Armitage P, McPherson CK, Rowe BC (1969) Repeated significance tests on accumulating data. J R Stat Soc Ser A 132:235–244
Armitage P (1991) Interim analysis in clinical trials. Stat Med 10:925–937
McPherson K (1974) The problem of examining accumulating data more than once. N Engl J Med 290:501–502
Wittes J, Schabenberger O, Zucker D, Brittain D, Proschan M (1999) Internal pilot studies I: type I error rate of the naive t-test. Stat Med 18:3481–3491
Coffey CS, Muller KE (2001) Controlling test size while gaining the benefits of an internal pilot design. Biometrics 57:625–631
Potvin D, DiLiberti CE, Hauck WW, Parr AF, Schuirmann DJ, Smith RA (2008) Sequential design approaches for bioequivalence studies with crossover designs. Pharm Stat 7:245–262. doi:10.1002/pst.294
Wonnemann M, Frömke C, Koch A (2014) Inflation of the type I error: investigations on regulatory recommendations for bioequivalence of highly variable drugs. Pharm Res. doi:10.1007/s11095-014-1450-z
National Institute of Health Science, Division of Drugs (2012) Guideline for bioequivalence studies of generic products. http://www.nihs.go.jp/drug/be-guide(e)/Generic/GL-E_120229_BE.pdf Accessed 27 October 2014
World Health Organization Expert Committee on Specifications for Pharmaceutical Preparations (2006) Annex 7. Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability. Fortieth Report, WHO Technical Report Series 937, Geneva. http://apps.who.int/prequal/info_general/documents/TRS937/WHO_TRS_937__annex7_eng.pdf Accessed 27 October 2014
National Institute of Health Science, Division of Drugs (2006) Guideline for bioequivalence studies of generic products. http://www.nihs.go.jp/drug/be-guide(e)/be2006e.pdf Accessed 27 October 2014
Korea Food & Drug Administration (2008) Guidance document for bioequivalence study. Doc. No. 11-1470000-001738-14
Estados Unidos Mexicanos, Secretaría de Salud (2013) Norma Oficial Mexicana NOM-177-SSA1-2013, Que establece las pruebas y procedimientos para demostrar que un medicamento es intercambiable. Diario Oficial, 20 September 2013. http://www.cofepris.gob.mx/AS/SiteAssets/Paginas/Ensayos%20Cl%C3%ADnicos/Temas/Marco-Jur%C3%ADdico/NOM-177-SSA1-2013.pdf Accessed 24 October 2014
Australian Department of Health, Therapeutic Goods Administration (2004) Australian regulatory guidelines for prescription medicines. Appendix 15: Biopharmaceutic Studies
Health Canada, Therapeutic Products Directorate (2009) Draft guidance document: conduct and analysis of comparative bioavailability studies. http://bebac.at/downloads/TPD%20Draft%20Guideline%20A+B%20Rev.%20V.2A.pdf Accessed 10 October 2014
Birkett MA, Day SJ (1994) Internal pilot studies for estimating sample size. Stat Med 13:2455–2463
Schuirmann D (2000) Nexium (Esomeprazole Magnesium) Delayed-release capsules. NDA 21–153. Statistical Review. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21154_Nexium_biopharmr_P2.pdf Accessed 26 October 2014
Pocock SJ (1977) Group sequential methods in the design and analysis of clinical trials. Biometrika 64:191–199
Pocock SJ (1982) Interim analyses for randomized clinical trials: the group sequential approach. Biometrics 38:153–162
Lan KG, DeMets DL (1983) Discrete sequential boundaries for clinical trials. Biometrika 70:659–663
Hauck WW, Preston PE, Bois FY (1997) A group sequential approach to crossover trials for average bioequivalence. J Biopharm Stat 71:87–96. doi:10.1080/10543409708835171
Jennison C, Turnbull BW (1999) Equivalence tests. In: Jennison C, Turnbull BW (eds) Group sequential methods with applications to clinical trials. Chapman & Hall/CRC, Boca Raton, pp 142–157
Gould AL (1995) Group sequential extension of a standard bioequivalence testing procedure. J Pharmacokinet Biopharm 23:57–86. doi:10.1007/BF02353786
Montague TH, Potvin D, DiLiberti CE, Hauck WW, Parr AF, Schuirmann DJ (2011) Additional results for ‘sequential design approaches for bioequivalence studies with crossover designs’. Pharm Stat 11:8–13. doi:10.1002/pst.483
Fuglsang A (2013) Sequential bioequivalence trial designs with increased power and controlled type I error rates. AAPS J 15:659–661. doi:10.1208/s12248-013-9475-5
Fuglsang A (2014) Sequential bioequivalence approaches for parallel designs. AAPS J 16:373–378. doi:10.1208/s12248-014-9571-1
Karalis V, Macheras P (2013) An insight into the properties of a two-stage design in bioequivalence studies. Pharm Res 30:1824–1835. doi:10.1007/s11095-013-1026-3
Karalis V (2013) The role of the upper sample size limit in two-stage bioequivalence designs. Int J Pharm 456:87–94. doi:10.1016/j.ijpharm.2013.08.013
Cui L, Hung HMJ, Wang S-J (1999) Modification of sample size in group sequential clinical trials. Biometrics 55:853–857
Fuglsang A (2014) Futility rules in bioequivalence trials with sequential designs. AAPS J 16:79–82. doi:10.1208/s12248-013-9540-0
Polli JE, Cook JA, Davit BM, Dickinson PA, Argenti D, Barbour N, García-Arieta A, Geoffroy J-M, Hartauer K, Li S, Mitra A, Muller FX, Purohit V, Sanchez-Felix M, Skoug JW, Tang K (2012) Summary workshop report: facilitating oral product development and reducing regulatory burden through novel approaches to assess bioavailability/bioequivalence. AAPS J 14:627–638. doi:10.1208/s12248-012-9376-z
Fuglsang A (2011) Controlling type I errors for two-stage bioequivalence study designs. Clin Res Regul Aff 28:100–105. doi:10.3109/10601333.2011.631547
Bauer P, Köhne K (1994) Evaluation of experiments with adaptive interim analyses. Biometrics 50:1029–1041
Lehmacher W, Wassmer G (1999) Adaptive sample size calculations in group sequential trials. Biometrics 55:1286–1290. doi:10.1111/j.0006-341X.1999.01286.x
Tsong Y, Zhang JJ, Wang SJ (2004) Group sequential design and analysis of clinical equivalence assessment for generic nonsystematic drug products. J Biopharm Stat 14:359–373. doi 10.1081/BIP-120037186
Friede T, Kieser M (2006) Sample size recalculation in internal pilot study designs: a review. Biomet J 48:537–555. doi:10.1002/bimj.200510238
Golkowski D, Friede T, Kieser M (2014) Blinded sample size re-estimation in crossover bioequivalence trials. Pharm Stat 13:157–162. doi:10.1002/pst.1617
Jones B, Kenward MG (2014) Chapters 12–14. In: Jones B, Kenward MG (eds) Design and analysis of cross-over trials, 3rd edn. Chapman & Hall/CRC, Boca Raton, pp 365–380
García-Arieta A, Gordon J (2012) Bioequivalence requirements in the European Union: critical discussion. AAPS J 14:738–748. doi:10.1208/s12248-012-9382-1
Zariffa NM-D, Patterson SD, Boyle D, Hyneck M (2000) Case studies, practical issues and observations on population and individual bioequivalence. Stat Med 19:2811–2820
European Medicines Agency, Committee for Human Medicinal Products (2013) Questions and answers: positions on specific questions addressed to the pharmacokinetics working party. EMA/618604/2008 Rev. 7. http://bebac.at/downloads/WC500002963Feb2013.pdf Accessed 22 October 2014
Karalis V, Macheras P (2014) On the statistical model of the two-stage designs in bioequivalence assessment. J Pharm Pharmacol 66:48–52. doi:10.1111/jphp.12164
O’Brien PC, Fleming TR (1979) A multiple testing procedure for clinical trials. Biometrics 35:549–556
Haybittle JL (1971) Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 44:793–797. doi:10.1259/0007-1285-44-526-793
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG (1977) Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 35:2–39. doi:10.1038/bjc.1977.1
European Generic Medicines Association (2010) Revised EMA bioequivalence guideline. questions and answers. Summary of the discussions held at the 3rd EGA Symposium on Bioequivalence. http://www.egagenerics.com/images/Website/EGA_BEQ_QA_WEB_QA_1_32.pdf Accessed 31 October 2014
Fuglsang A (2014) A sequential bioequivalence design with a potential ethical advantage. AAPS J 16:843–846. doi:10.1208/s12248-014-9622-7
Fenta HM (2014) Determination of sample size for two stage sequential designs in bioequivalence studies under 2 × 2 crossover design. Sci J Clin Med 3:82–90. doi:10.11648/j.sjcm.20140305.12
Bandyopadhyay N, Dragalin V (2007) Implementation of an adaptive group sequential design in a bioequivalence study. Pharm Stat 6:115–122. doi:10.1002/pst.252
Du D, Targett D, Stolberg E, Canali A (2013) A clinical pharmacokinetic study comparing two azelastine hydrochloride nasal formulations in a single-dose design. Eur J Drug Metab Pharmacokinet 39:69–75. doi:10.1007/s13318-013-0134-0
Zheng C, Wang J, Zhao L (2012) Testing bioequivalence for multiple formulations with power and sample size calculations. Pharm Stat 2012:334–341. doi:10.1002/pst.1522
Jennison C, Turnbull BW(2003) Mid-course sample size modification in clinical trials based on the observed treatment effect. Stat Med 22:971–993. doi:10.1002/sim.1457
Tsiatis AA, Mehta C (2003) On the inefficiency of the adaptive design for monitoring clinical trials. Biometrika 90:367–378. doi:10.1093/biomet/90.2.367
Acknowledgments
The author would like to thank Anders Fuglsang for providing the C-code and Detlew Labes for the fruitful discussions.
Conflict of interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 79 kb)
Rights and permissions
About this article
Cite this article
Schütz, H. Two-stage designs in bioequivalence trials. Eur J Clin Pharmacol 71, 271–281 (2015). https://doi.org/10.1007/s00228-015-1806-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1806-2