Abstract
Purpose
In absence of specific dosing guidelines, the optimal dose of low molecular weight heparins for thrombosis prophylaxis in morbidly obese patients (BMI > 40 kg/m2) remains unknown. In order to guide dosing in this patient group, a pharmacodynamics model is developed for nadroparin in morbidly obese and non-obese patients using anti-Xa levels as an endpoint, thereby characterizing the influence of excessive body weight on different pharmacodynamic model parameters.
Methods
Twenty-eight morbidly obese and seven non-obese patients receiving 5700 IU and 2850 IU subcutaneous (s.c.) nadroparin for surgery, respectively, were included with a mean total body weight (TBW) of 135 kg (range 72–252 kg). Up to 11 anti-Xa levels were collected from the start until 24 h after nadroparin administration. Population pharmacodynamic modelling with covariate analysis was performed using NONMEM.
Results
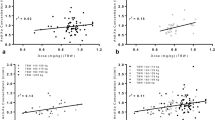
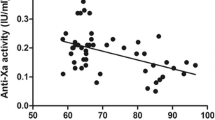
In a two-compartment pharmacodynamic model with baseline endogenous anti-Xa levels, the effect of nadroparin was found to be delayed and could be best described using a transit compartment. TBW was the most predictive covariate for clearance (CL = 23.0 mL/min × (TBW/70)), while lean body weight (LBW) proved the most predictive covariate for central volume of distribution (V1 = 7.0 L × (LBW/60)).
Conclusions
A pharmacodynamic model was developed characterizing anti-Xa levels after s.c. administration of nadroparin in patients weighing between 72 and 252 kg with TBW and LBW as the major determinants for clearance and volume of distribution, respectively.





Similar content being viewed by others
References
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373:1083–1096
World Health Organisation (1997) Obesity: preventing and managing the global epidemic. World Health Organisation, Geneva
Stein PD, Beemath A, Olson RE (2005) Obesity as a risk factor in venous thromboembolism. Am J Med 118:978–980
Stein PD, Matta F, Goldman J (2011) Obesity and pulmonary embolism: the mounting evidence of risk and the mortality paradox. Thromb Res 128:518–523
Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH (2012) Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141:e152S–e184S
Rondina MT, Wheeler M, Rodgers GM, Draper L, Pendleton RC (2010) Weight-based dosing of enoxaparin for VTE prophylaxis in morbidly obese, medically-ill patients. Thromb Res 125:220–223
Singh K, Podolsky ER, Um S, Saba S, Saeed I, Aggarwal L, Zaya M, Castellanos A (2012) Evaluating the safety and efficacy of BMI-based preoperative administration of low-molecular-weight heparin in morbidly obese patients undergoing Roux-en-Y gastric bypass surgery. Obes Surg 22:47–51
Borkgren-Okonek MJ, Hart RW, Pantano JE, Rantis PC Jr, Guske PJ, Kane JM Jr, Gordon N, Sambol NC (2008) Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis 4:625–631
Kalfarentzos F, Stavropoulou F, Yarmenitis S, Kehagias I, Karamesini M, Dimitrakopoulos A, Maniati A (2001) Prophylaxis of venous thromboembolism using two different doses of low-molecular-weight heparin (nadroparin) in bariatric surgery: a prospective randomized trial. Obes Surg 11:670–676
Kiang TK, Sherwin CM, Spigarelli MG, Ensom MH (2012) Fundamentals of population pharmacokinetic modelling: modelling and software. Clin Pharmacokinet 51:515–525
Duffull SB, Wright DF (2013) What do we learn from repeated population analyses? Br J Clin Pharmacol
Barras MA, Duffull SB, Atherton JJ, Green B (2009) Modelling the occurrence and severity of enoxaparin-induced bleeding and bruising events. Br J Clin Pharmacol 68:700–711
Hulot JS, Vantelon C, Urien S, Bouzamondo A, Mahe I, Ankri A, Montalescot G, Lechat P (2004) Effect of renal function on the pharmacokinetics of enoxaparin and consequences on dose adjustment. Ther Drug Monit 26:305–310
Hulot JS, Montalescot G, Lechat P, Collet JP, Ankri A, Urien S (2005) Dosing strategy in patients with renal failure receiving enoxaparin for the treatment of non-ST-segment elevation acute coronary syndrome. Clin Pharmacol Ther 77:542–552
Green B, Greenwood M, Saltissi D, Westhuyzen J, Kluver L, Rowell J, Atherton J (2005) Dosing strategy for enoxaparin in patients with renal impairment presenting with acute coronary syndromes. Br J Clin Pharmacol 59:281–290
Berges A, Laporte S, Epinat M, Zufferey P, Alamartine E, Tranchand B, Decousus H, Mismetti P (2007) Anti-factor Xa activity of enoxaparin administered at prophylactic dosage to patients over 75 years old. Br J Clin Pharmacol 64:428–438
Green B, Duffull SB (2003) Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol 56:96–103
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B (2005) Quantification of lean bodyweight. Clin Pharmacokinet 44:1051–1065
Diepstraten J, Hackeng CM, Van Kralingen S, Zapletal J, Van Dongen EP, Wiezer MJ, Ramshorst B, Knibbe CA.(2012) Anti-Xa levels 4 hours after subcutaneous administration of 5700 IU nadroparin strongly correlate with lean body weight in morbidly obese patients. Obes Surg
Beal SL, Sheiner LB, Boeckmann A (1999) NONMEM user’s guide. University of California, San Francisco
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82:17–20
Comets E, Brendel K, Mentre F (2008) Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 90:154–166
Savic RM, Jonker DM, Kerbusch T, Karlsson MO (2007) Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34:711–726
Schoemaker RC, Cohen AF (1996) Estimating impossible curves using NONMEM. Br J Clin Pharmacol 42:283–290
Barrett JS, Gibiansky E, Hull RD, Planes A, Pentikis H, Hainer JW, Hua TA, Gastonguay M (2001) Population pharmacodynamics in patients receiving tinzaparin for the prevention and treatment of deep vein thrombosis. Int J Clin Pharmacol Ther 39:431–446
Pai MP, Paloucek FP (2000) The origin of the "ideal" body weight equations. Ann Pharmacother 34:1066–1069
Anderson BJ, Holford NH (2009) Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 24:25–36
Nutescu EA, Spinler SA, Wittkowsky A, Dager WE (2009) Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother 43:1064–1083
Savic RM, Karlsson MO (2009) Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J 11:558–569
Lemmens HJ, Bernstein DP, Brodsky JB (2006) Estimating blood volume in obese and morbidly obese patients. Obes Surg 16:773–776
Barras MA, Duffull SB, Atherton JJ, Green B (2008) Individualized compared with conventional dosing of enoxaparin. Clin Pharmacol Ther 83:882–888
Feng Y, Green B, Duffull SB, Kane-Gill SL, Bobek MB, Bies RR (2006) Development of a dosage strategy in patients receiving enoxaparin by continuous intravenous infusion using modelling and simulation. Br J Clin Pharmacol 62:165–176
Green B, Duffull S (2002) Caution when lean body weight is used as a size descriptor for obese subjects. Clin Pharmacol Ther 72:743–744
Eleveld DJ, Proost JH, Absalom AR, Struys MM (2011) Obesity and allometric scaling of pharmacokinetics. Clin Pharmacokinet 50:751–753
Samama MM, Gerotziafas GT (2000) Comparative pharmacokinetics of LMWHs. Semin Thromb Hemost 26(Suppl 1):31–38
Bourin MC, Lindahl U (1993) Glycosaminoglycans and the regulation of blood coagulation. Biochem J 289(Pt 2):313–330
Hainer JW, Barrett JS, Assaid CA, Fossler MJ, Cox DS, Leathers T, Leese PT (2002) Dosing in heavy-weight/obese patients with the LMWH, tinzaparin: a pharmacodynamic study. Thromb Haemost 87:817–823
Siguret V, Pautas E, Fevrier M, Wipff C, Durand-Gasselin B, Laurent M, Andreux JP, d’Urso M, Gaussem P (2000) Elderly patients treated with tinzaparin (Innohep) administered once daily (175 anti-Xa IU/kg): anti-Xa and anti-IIa activities over 10 days. Thromb Haemost 84:800–804
Harenberg J, Jeschek M, Acker M, Malsch R, Huhle G, Heene DL (1996) Effects of low-molecular-weight dermatan sulfate on coagulation, fibrinolysis and tissue factor pathway inhibitor in healthy volunteers. Blood Coagul Fibrinolysis 7:49–56
Frydman A (1996) Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis 26(Suppl 2):24–38
Sanderink GJ, Le Liboux A, Jariwala N, Harding N, Ozoux ML, Shukla U, Montay G, Boutouyrie B, Miro A (2002) The pharmacokinetics and pharmacodynamics of enoxaparin in obese volunteers. Clin Pharmacol Ther 72:308–318
Ribstein J, du Cailar G, Mimran A (1995) Combined renal effects of overweight and hypertension. Hypertension 26:610–615
Hirsh J, Raschke R (2004) Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126:188S–203S
Harenberg J (2004) Is laboratory monitoring of low-molecular-weight heparin therapy necessary? Yes. J Thromb Haemost 2:547–550
Paige JT, Gouda BP, Gaitor-Stampley V, Scalia PG, Klainer TE, Raum WJ, Martin LF (2007) No correlation between anti-factor Xa levels, low-molecular-weight heparin, and bleeding after gastric bypass. Surg Obes Relat Dis 3:469–475
Bounameaux H, de Moerloose P (2004) Is laboratory monitoring of low-molecular-weight heparin therapy necessary? No. J Thromb Haemost 2:551–554
Acknowledgments
The authors would like to thank Koos Dubbeldam for the laboratory assessments and Brigitte Bliemer and Silvia Samson for their enthusiastic support in this study.
Contributions of authors statement
Jeroen Diepstraten executed the study, participated in the design and informed consent, analysed data and wrote manuscript. Esther J.H. Janssen executed the study and participated in informed consent and analysing the data. Christian M. Hackeng analysed the blood samples and participated in the design and preparation of the manuscript. Eric P.A. van Dongen participated in the design, informed consent and preparation of the manuscript. René J. Wiezer participated in the design, informed consent and preparation of the manuscript. Bert van Ramshorst was the principal investigator who designed and supervised the study and participated in analysing the data and preparation of the manuscript. Catherijne A.J. Knibbe was the principal investigator who designed, supervised the study, analysed data and participated in preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diepstraten, J., Janssen, E.J.H., Hackeng, C.M. et al. Population pharmacodynamic model for low molecular weight heparin nadroparin in morbidly obese and non-obese patients using anti-Xa levels as endpoint. Eur J Clin Pharmacol 71, 25–34 (2015). https://doi.org/10.1007/s00228-014-1760-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1760-4