Abstract
Purpose
Prevalence data on the off-label use (OLU) of anticancer drugs are limited despite OLU being controversial for medical, pharmaco-economic, and ethical reasons. We therefore quantified and characterized the OLU of anticancer drugs and compared OLU based on the national drug label with international treatment recommendations.
Methods
We prospectively collected data on patients receiving systemic anticancer therapy between October and December 2012 at hospitals affiliated with the Eastern Switzerland Oncology Network. Individual data on patient characteristics, tumor disease, and systemic treatment were collected, and each individual treatment was compared with the national drug label and international treatment guidelines.
Results
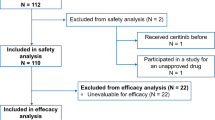
A total of 985 consecutive patients receiving 1,737 anticancer drug treatments were included in the study. Overall, 32.4 % of all patients received at least one off-label drug, corresponding to 27.2 % of all anticancer drugs administered. Major reasons for OLU were the lack of approval for the specific disease entity (15.7 %) and modified application of the anticancer drug (10 %). OLU that was unsupported by the current European Society for Medical Oncology (ESMO) treatment recommendations was rare (6.6 %) but higher for bevacizumab (29.6 %) due to its use in treating advanced ovarian cancer beyond the second-line setting and advanced breast cancer beyond the first-line setting and for lenalidomide (22.6 %) due to its use in treating Non-Hodgkin lymphoma.
Conclusions
Based on data collected on our patient cohort, OLU of anticancer drugs in a European clinical setting applies to one-third of all cancer patients. ESMO-unsupported use of chemotherapies or molecularly-targeted drugs is rare, opposing concerns that the off-label use of newer anticancer drugs is a substantial clinical problem.



Similar content being viewed by others
References
Bach PB (2009) Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med 360(6):626–633. doi:10.1056/NEJMhpr0807774
Conti RM, Bernstein AC, Villaflor VM, Schilsky RL, Rosenthal MB, Bach PB (2013) Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologists. J Clin Oncol 31(9):1134–1139. doi:10.1200/JCO.2012.42.7252
Radley DC, Finkelstein SN, Stafford RS (2006) Off-label prescribing among office-based physicians. Arch Intern Med 166(9):1021–1026. doi:10.1001/archinte.166.9.1021
Leveque D (2008) Off-label use of anticancer drugs. Lancet Oncol 9:1102–1107
Laetz T, Silberman G (1991) Reimbursement policies constrain the practice of oncology. JAMA 266(21):2996–2999
Shrank WH, Asch SM, Adams J, Setodji C, Kerr EA, Keesey J, Malik S, McGlynn EA (2006) The quality of pharmacologic care for adults in the United States. Med Care 44(10):936–945. doi:10.1097/01.mlr.0000223460.60033.79
Swissmedic (Swiss Agency for Therapeutic Products). Available at: http://www.swissmedicinfo.ch. Last accessed 16 Feb 2014
Glimelius B, Tiret E, Cervantes A, Arnold D, Group EGW (2013) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol[Suppl 6]:vi81–88. doi:10.1093/annonc/mdt240
Group EESNW (2012) Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol[Suppl 7]:vii92–99. doi:10.1093/annonc/mds253
Horwich A, Parker C, de Reijke T, Kataja V, Group EGW (2013) Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24[Suppl 6]:vi106–114. doi:10.1093/annonc/mdt208
Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, Arnold D, Group EGW (2013) Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24[Suppl 6]:vi64–72. doi:10.1093/annonc/mdt354
Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Kohne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 23(10):2479–2516. doi:10.1093/annonc/mds236
Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E, Group EGW (2012) Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol[Suppl 7]:vii56–64. doi:10.1093/annonc/mds226
Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D, Group EGW (2013) Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol[Suppl 6]:vi51–56. doi:10.1093/annonc/mdt342
National Comprehensive Cancer Network (NCCN) (2013) NCCN guidelines. Available at: http://www.nccnorg/professionals/defaultaspx
Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, Group EGW (2013) Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24[Suppl 6]:vi24–32. doi:10.1093/annonc/mdt333
American Society of Clinical Oncology (2009) Recent developments in Medicare coverage of off-label cancer therapies. J Oncol Pract (1):18–20
Aitken M, Berndt ER, Cutler DM (2009) Prescription drug spending trends in the United States: looking beyond the turning point. Health Aff 28(1):w151–160. doi:10.1377/hlthaff.28.1.w151
American Society of Clinical Oncology (2006) Reimbursement for cancer treatment: coverage of off-label drug indications. J Clin Oncol 24(19):3206–3208
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O'Neill V, Rugo HS (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29(32):4286–4293. doi:10.1200/JCO.2010.34.1255
Verschraegen CF, Czok S, Muller CY, Boyd L, Lee SJ, Rutledge T, Blank S, Pothuri B, Eberhardt S, Muggia F (2012) Phase II study of bevacizumab with liposomal doxorubicin for patients with platinum- and taxane-resistant ovarian cancer. Ann Oncol 23(12):3104–3110. doi:10.1093/annonc/mds172
Colucci G, Labianca R, Di Costanzo F, Gebbia V, Carteni G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolo M, Ciaparrone M, Cavanna L, Giuliani F, Maiello E, Testa A, Pederzoli P, Falconi M, Gallo C, Di Maio M, Perrone F, Gruppo Oncologico Italia M, Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato D, Gruppo Oncologico Italiano di Ricerca C (2010) Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 28(10):1645–1651. doi:10.1200/JCO.2009.25.4433
Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D, Wenham R, McGuire W (2007) Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 25(33):5180–5186. doi:10.1200/JCO.2007.12.0782
Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI (2007) Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25(33):5165–5171. doi:10.1200/JCO.2007.11.5345
Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, Bouabdallah R, Haioun C, Tilly H, Guo P, Pietronigro D, Ervin-Haynes AL, Czuczman MS (2011) An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol 22(7):1622–1627. doi:10.1093/annonc/mdq626
Tilly H, Vitolo U, Walewski J, da Silva MG, Shpilberg O, Andre M, Pfreundschuh M, Dreyling M, Group EGW (2012) Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23[Suppl 7[:vii78–82. doi:10.1093/annonc/mds273
Piccart MJ, Bertelsen K, Stuart G, Cassidy J, Mangioni C, Simonsen E, James K, Kaye S, Vergote I, Blom R, Grimshaw R, Atkinson R, Swenerton K, Trope C, Nardi M, Kaern J, Tumolo S, Timmers P, Roy JA, Lhoas F, Lidvall B, Bacon M, Birt A, Andersen J, Zee B, Paul J, Pecorelli S, Baron B, McGuire W (2003) Long-term follow-up confirms a survival advantage of the paclitaxel-cisplatin regimen over the cyclophosphamide-cisplatin combination in advanced ovarian cancer. Int J Gynecol Cancer 13[Suppl 2]:144–148
Bramwell VH, Mouridsen HT, Santoro A, Blackledge G, Somers R, Verweij J, Dombernowsky P, Onsrud M, Thomas D, Sylvester R et al (1993) Cyclophosphamide versus ifosfamide: a randomized phase II trial in adult soft-tissue sarcomas. The European Organization for Research and Treatment of Cancer [EORTC], Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharm 31[Suppl 2]:S180–184
Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, Fanucchi M, Harmon DC, Schuetze SM, Reinke D, Thall PF, Benjamin RS, Baker LH, Hensley ML (2007) Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25(19):2755–2763. doi:10.1200/JCO.2006.10.4117
Evans D, Miner T, Iannitti D, Akerman P, Cruff D, Maia-Acuna C, Harrington D, Habr F, Chauhan B, Berkenblit A, Stuart K, Sears D, Kennedy T, Safran H (2007) Docetaxel, capecitabine and carboplatin in metastatic esophagogastric cancer: a phase II study. Cancer Investig 25(6):445–448. doi:10.1080/07357900701358025
Chang HM, Kim TW, Ryu BY, Choi SJ, Park YH, Lee JS, Kim WK, Kang YK (2005) Phase II study of paclitaxel and carboplatin in advanced gastric cancer previously treated with 5-fluorouracil and platinum. Jpn J Clin Oncol 35(5):251–255. doi:10.1093/jjco/hyi077
Valentini V, Coco C, Minsky BD, Gambacorta MA, Cosimelli M, Bellavita R, Morganti AG, La Torre G, Trodella L, Genovesi D, Portaluri M, Maurizi-Enrici R, Barbera F, Maranzano E, Lupattelli M (2008) Randomized, multicenter, phase IIb study of preoperative chemoradiotherapy in T3 mid-distal rectal cancer: raltitrexed + oxaliplatin + radiotherapy versus cisplatin + 5-fluorouracil + radiotherapy. Int J Radiat Oncol Biol Phys 70(2):403–412. doi:10.1016/j.ijrobp.2007.06.025
Roila F, Ballatori E, Labianca R, De Braud F, Borgonovo K, Martelli O, Gallo C, Tinazzi A, Perrone F; Italian Medical Oncology Association (2009) Off-label prescription of antineoplastic drugs: an Italian prospective, observational, multicenter survey. Tumori 95(6):647–651
Gota V, Patial P (2011) Off-label use of anti-cancer drugs in India: to be or not to be! J Cancer Res Ther 7(1):35–39. doi:10.4103/0973-1482.80455
Casali PG (2007) The off-label use of drugs in oncology: a position paper by the European Society for Medical Oncology (ESMO). Ann Oncol 18:1923–1925
Liang Y, Zhang L, Gao J, Hu D, Ai Y (2012) Rituximab for children with immune thrombocytopenia: a systematic review. PloS one 7(5):e36698. doi:10.1371/journal.pone.0036698
Davies JE, Neidle S, Taylor DG (2012) Developing and paying for medicines for orphan indications in oncology: utilitarian regulation vs equitable care? Br J Cancer 106(1):14–17. doi:10.1038/bjc.2011.544
Schilsky RL (2013) Publicly funded clinical trials and the future of cancer care. Oncologist 18(2):232–238. doi:10.1634/theoncologist.2012-0423
Acknowledgments
The study has been supported by the Swiss Cancer League.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joerger, M., Schaer-Thuer, C., Koeberle, D. et al. Off-label use of anticancer drugs in eastern Switzerland: a population-based prospective cohort study. Eur J Clin Pharmacol 70, 719–725 (2014). https://doi.org/10.1007/s00228-014-1662-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1662-5