Abstract
Objective
Inhaled corticosteroids may cause oropharyngeal side effects if deposited in the oropharynx in active form. Ciclesonide, an inhaled corticosteroid with low glucocorticoid receptor affinity, is activated primarily in the lung by esterases to an active metabolite, desisobutyryl-ciclesonide (des-CIC), with high glucocorticoid receptor affinity. We studied oropharyngeal deposition of ciclesonide, des-CIC, and budesonide.
Methods
In an open-label, randomized, two-treatment (administered in sequence), five-period study, 18 healthy subjects received 800 μg (ex-valve) inhaled ciclesonide via a hydrofluoroalkane-pressurized, metered-dose inhaler followed by 800 μg budesonide (Pulmicort) by a chlorofluorocarbon-pressurized, metered-dose inhaler (four puffs of 200 μg each, ex-valve) or vice versa. Oropharyngeal cavity rinsing was performed immediately, or 15, 30, 45, or 60 min after inhalation (one rinsing per study period), and the solutions were analyzed using liquid chromatography with tandem mass spectrometric detection.
Results
Ciclesonide and budesonide were detected in most oropharyngeal wash samples. Maximal concentration of each inhaled corticosteroid was reached immediately post-inhalation; maximal concentrations of ciclesonide and des-CIC were 30% and 0.67%, respectively, of budesonide. Oropharyngeal deposition of ciclesonide and budesonide decreased rapidly within 15 min post-inhalation, and less rapidly thereafter. Less than 10% of the residual ciclesonide in the oropharynx was converted to des-CIC. The molar dose-adjusted amount of des-CIC was 4% of budesonide (P < 0.0001). There were no significant adverse events.
Conclusion
Oropharyngeal deposition of des-CIC was more than one order of magnitude lower than that of budesonide when administered by the respective metered-dose inhalers. This may explain the low frequency of oropharyngeal side effects of ciclesonide in clinical studies.
Similar content being viewed by others
Introduction
Inhaled corticosteroids (ICs) are the anti-inflammatory treatment of choice for persistent asthma. The therapeutic effect of these agents depends on the degree of pulmonary deposition and affinity for glucocorticoid receptors. ICs can be deposited in the oropharyngeal cavity, regardless of device, potentially leading to local complications such as hoarseness (dysphonia), pharyngitis, and oral candidiasis [11]. Furthermore, corticosteroids deposited in the oropharynx may be swallowed and absorbed into the systemic circulation, possibly resulting in suppression of cortisol release and disturbances in bone metabolism and growth [19].
Systemic exposure to ICs depends on their pulmonary and oral bioavailability, the latter of which ranges from less than 1% to 26% [21]. For example, the oral bioavailability of budesonide is approximately 11%, meaning that greater than one-tenth of the swallowed drug can be detected in the systemic circulation. However, oropharyngeal deposition of budesonide depends on the inhalation device. When budesonide was administered through a pressurized metered-dose inhaler (pMDI), the contribution from the swallowed drug to the overall systemic availability was 42% [26]. Because of the high potential for currently available ICs to produce local and systemic complications, there is a clear need for ICs with an improved safety profile.
Ciclesonide (Alvesco; ALTANA Pharma AG, Konstanz, Germany) is a nonhalogenated IC that is formulated as a solution for use in a hydrofluoroalkane (HFA)-pMDI. The HFA-pMDI provides a fine particle spray, thereby yielding high pulmonary deposition in central and peripheral regions of the lung and minimizing oropharyngeal deposition. The oral bioavailability of ciclesonide is less than 1% [15]. Ciclesonide, administered as a parent compound, is converted by pulmonary esterases to an active metabolite, desisobutyryl-ciclesonide (des-CIC), which has a 100-fold greater relative glucocorticoid receptor binding affinity than the ciclesonide parent compound (relative glucocorticoid receptor binding affinities are 1,212 and 12, respectively; dexamethasone reference is 100), while budesonide has a relative receptor binding affinity of 905 [24]. Because ciclesonide is inactive when inhaled, the likelihood of side effects in the mouth or upper respiratory tract is reduced [11]. This study compared the oropharyngeal deposition of ciclesonide and des-CIC versus that of budesonide. Ciclesonide and budesonide were administered as successive inhalations using comparable delivery devices, an HFA-pMDI and a chlorofluorocarbon (CFC)-pMDI, respectively. However, formulation of budesonide as a suspension in the pMDI is likely to produce a coarser particle spray and to increase oropharyngeal deposition.
Methods
Subjects
Healthy subjects, 18–65 years of age, of normal weight according to the Broca Index (0.80≤weight/[height−100]≤1.25), and with stable smoking habits were eligible. A medical history and physical examination were performed during a screening visit within 4 weeks of the start of the study. Subjects had to be able to rinse their mouths and gargle with two fractions of 30 ml 50% (vol/vol) ethanol. Subjects were excluded if they had any active oropharyngeal disorder; had clinically relevant allergies; had taken medication within 2 weeks before study entry; had been screened for human immunodeficiency virus or hepatitis; had a positive drug test; or had abused alcohol or drugs. Women were excluded if they did not use a reliable form of contraception or were pregnant. Subjects provided written informed consent. This study was performed in accordance with Good Clinical Practice and was approved by an independent institutional review board.
Study design and treatment
This was an open-label, two-treatment, five-period study with random allocation of eligible subjects to two inhalation sequences (Table 1). Each subject inhaled ciclesonide 800 μg by HFA-pMDI (four puffs of 200 μg each, ex-valve) followed by budesonide 800 μg by CFC-pMDI (Pulmicort; four puffs of 200 μg each, ex-valve), or budesonide followed by ciclesonide at the same doses. The corresponding molar doses were 1.48 μmol and 1.86 μmol for ciclesonide and budesonide, respectively. In each of the five study periods, each patient received the medications as a single dose in the same sequence (over a period of a few minutes) and at the same time of day as in the original allocation (Table 1). Each study period was separated by a washout period of approximately 24 h, and each subject usually completed the study in the same week.
Oropharyngeal washing
In order to recover study drug deposited in the oropharynx, an oropharyngeal wash of 30 ml of 50% (vol/vol) ethanol was performed immediately or 15, 30, 45, or 60 min after inhalation for study periods 1–5, respectively. Each patient rinsed his or her mouth for 5 s and gargled for 2–3 s, and the solution was recovered. The oropharyngeal washing step was repeated and the two samples were pooled. A 10-ml aliquot was withdrawn from the pooled sample and stored at −20°C until bioanalytical analysis was performed.
Assessments
The primary variable of the study, on a molar basis, was the respective area under the concentration time curve between 0 min and 60 min (AUC0–60 min) of ciclesonide, des-CIC, and budesonide in the rinsing solution. The AUC0–60 min was calculated by the trapezoidal formula. Other pharmacokinetic parameters were the maximum drug concentration in the rinsing solution (Cmax) and the time at which Cmax was achieved (tmax). Adverse events were monitored continuously during the study.
Budesonide and ciclesonide concentrations were determined simultaneously in rinsing solutions using an internal standard and flow injection liquid chromatography with tandem mass spectrometric detection (LC/MS/MS) (PE-Sciex API3000, Quest Pharmaceutical Services, Newark, DE). Briefly, 0.2 ml of each sample was mixed with 0.2 ml of internal standard and 4 ml of the mobile phase (850 ml methanol, 100 ml Millipore water, and 50 ml 25 mM ammonium acetate). Following centrifugation, a 5-μl aliquot of the solution was injected into the LC/MS apparatus using a mobile phase at a flow rate of 0.1 ml/min. To determine the concentration of des-CIC, mouth-rinsing samples were spiked with an internal standard, and 5 μl was injected into a reversed-phase LC/MS/MS system (Waters Symmetry C18, 3.5 μm, 2.1×50 mm) at a flow rate of 0.2 ml/min. Analytes of interest were detected using a PE-Sciex API3000 in negative ion daughter mode.
The concentrations of unknown and quality control samples were determined by linear least-squares regression by plotting the peak area ratios of ciclesonide, des-CIC, and budesonide to the corresponding internal standard against the nominal concentrations of ciclesonide, des-CIC, and budesonide. Quantification was performed using MacQuan 1.6 software (PerkinElmer Sciex, Orlando, FL). Calibration ranges were 1–50 ng/ml for des-CIC and 10–500 ng/ml for ciclesonide and budesonide. Calibration, quality control data, and chromatograms demonstrated the precision, accuracy, and between-batch reproducibility of the methods. The within-batch accuracy for ciclesonide, budesonide, and des-CIC were in the range of 95.1–109.7%, 96.6–109.8%, and 97.8–104.8%, respectively. Between-batch precision values, based on the coefficient of variation of quality-control samples, were 3.2% or less for all three analytes.
Statistical analyses
Point estimates and 95% confidence limits were calculated for the ratios of the molar AUC population medians of des-CIC and budesonide as primary analysis, ciclesonide plus des-CIC and budesonide, and des-CIC and ciclesonide as secondary analyses. A multiplicative model, reflecting the two-treatment sequences, and a parametric analysis after logarithmic transformation (including molar adjustment of 800 μg ciclesonide to 1.48 μmol and 800 μg budesonide to 1.86 μmol) were performed. The secondary variables were analyzed in a descriptive manner using summary statistics. Statistical significance was based on the 95% confidence limits. Finally, a two-sided t-test (Wilcoxon–Pratt test) was performed to assign the significance of the comparisons.
Results
Eighteen subjects (9 men and 9 women), with a median age of 33 years (range, 22–55 years), were enrolled in this study. All subjects were caucasian and of normal weight (median Broca Index, 98%). The mean volume of recovered rinsing solution was 56 ml (of the 60 ml used). Because of the relatively small coefficient of variation (4.7%), compound concentrations were not adjusted according to the volume of rinsing solution recovered. The treatment sequence did not affect the volume of recovered mouth-rinsing solution.
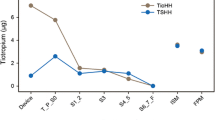
Maximum concentrations of ciclesonide and budesonide were attained in the rinsing solutions immediately after inhalation. The mean Cmax of ciclesonide was 1.8 mg/l (3.3 μmol/l) and was 70.5% lower than the Cmax of budesonide, which was 4.8 mg/l (11.3 μmol/l) (Table 2). Total mean amounts of 100.8 μg of ciclesonide and 268.8 μg of budesonide were recovered in mouth-rinsing solutions collected at the first available time point (2–5 min). These amounts corresponded to 12.6% and 33.6% of the nominal inhaled doses of 800 μg of ciclesonide and budesonide, respectively. Ciclesonide and budesonide concentrations in the rinsing solutions decreased rapidly during the 15 min following administration and less rapidly thereafter (Fig. 1). For both ciclesonide and budesonide, Cmax was achieved at a similar time point after inhalation (median tmax=2.5 min or 0.05 h). In contrast, the concentration of des-CIC at the first available time point was 0.005 mg/l (10.3 nmol/l) and increased slightly over time to achieve a mean Cmax of 0.037 mg/l (76 nmol/l) after 0.74 h (tmax). The Cmax of des-CIC was 0.67% of that of budesonide. Concentrations of ciclesonide plus des-CIC in rinsing solutions were numerically less than those of budesonide at all evaluation time points. The mean amount of des-CIC at tmax corresponds to 4.3 nmol des-CIC and represents 0.3% of the nominal inhaled dose of 1.48 μmol ciclesonide.
Oropharyngeal deposition of ciclesonide, desisobutyryl-ciclesonide (des-CIC), and budesonide. Mean (SEM) concentrations of study drugs in the rinsing solutions of 18 healthy patients following inhalation of 800 μg ciclesonide and 800 μg budesonide delivered via HFA-pMDI and CFC-pMDI, respectively. A Comparison of ciclesonide (filled triangle), des-CIC (filled circle), and budesonide (filled square). B Comparison of budesonide (filled square), ciclesonide plus des-CIC (open circle), and des-CIC (filled circle)
The molar AUC0–60 min was calculated for ciclesonide, des-CIC, and budesonide to allow direct comparisons between compounds. Activation of ciclesonide to des-CIC within the oropharynx was very low and occurred slowly. Based on molar AUC0–60 min values, the amount of des-CIC detected in the rinsing solution was 8% of that of ciclesonide (Table 2). Oropharyngeal deposition of des-CIC was low compared with budesonide. Molar dose-adjusted AUC0–60 min values revealed that the amount of des-CIC detected in the rinsing solution was 4% of that of budesonide (Table 3 and Fig. 2). This difference between des-CIC and budesonide was statistically significant (95% CI 0.02, 0.05; P<0.0001). Oropharyngeal deposition of ciclesonide plus des-CIC was low compared with budesonide. The molar amount of ciclesonide plus des-CIC detected in the rinsing solution within the first hour following inhalation was 47% of that of budesonide. This difference was also statistically significant (95% CI 0.38, 0.59; P<0.0001).
Both ciclesonide and budesonide were well tolerated when administered in sequence. No oropharyngeal adverse events occurred after treatment with either agent. Three cases of headache and one case of herpes facialis were reported and assessed to be unrelated to the study medication. No deaths, other serious adverse events, or clinically significant abnormalities occurred during the study.
Discussion
ICs are standard in the care of patients with persistent asthma. However, some of the currently available ICs are associated with oropharyngeal deposition and accompanying side effects. The incidence of oropharyngeal side effects depends on the IC dose, frequency of administration, and delivery system [11]. Voice hoarseness is the most frequent local side effect associated with IC use and is alleviated by treatment withdrawal, which may compromise effective asthma therapy. Williamson et al. [30] found that 58% of patients receiving ICs reported throat symptoms or dysphonia compared with 13% of control patients. Local side effects were equally prevalent among patients treated with beclomethasone dipropionate and budesonide [30]. The incidence of oropharyngeal candidiasis is correlated with both dose and dosing frequency of budesonide in asthmatic adults [27, 28]. However, few clinical trials have systematically assessed the local side effects of ICs.
An IC administered in its active form is more likely to cause local side effects [11]. Ciclesonide is a novel IC that is inactive until it is delivered to the lungs where cleavage by esterases generates the active metabolite [1]. Furthermore, ciclesonide is highly protein bound [21] and is rapidly metabolized in the liver [1, 16, 22] resulting in a compound with low bioavailability [15] and reduced capacity to cause systemic effects. Previous trials have demonstrated that the incidence of ciclesonide-associated oropharyngeal side effects is low [6, 8, 20, 25]. Furthermore, clinical trials have shown that ciclesonide is not associated with cortisol suppression [29].
Several studies have demonstrated the clinical efficacy of ciclesonide in patients with asthma at doses at or below the 800-μg dose evaluated in this study [6, 20, 25]. The results of the current study demonstrate that a clinically effective dose of ciclesonide has a significantly lower level of oropharyngeal deposition than budesonide. On a nanomolar dose-adjusted basis, oropharyngeal deposition of ciclesonide and des-CIC was only 47% of budesonide deposition, and this difference is likely related to the different inhaler devices. Previous studies have shown that the HFA-pMDI produces an IC with a smaller particle size than the CFC-pMDI [13]. Consequently, greater IC pulmonary deposition is achieved using a HFA-pMDI than a CFC-pMDI. Budesonide has an average particle size of 10.2 μm, and approximately 17% of a 200-μg dose of budesonide is respirable [2]. Conversely, the average particle size of ciclesonide is 1.1–2.1 μm, and approximately 48% of a 200-μg dose of ciclesonide is respirable [21, 23]. High lung deposition of an IC is correlated with low oropharyngeal deposition [18, 19]. In two previous studies using 2D and 3D scintigraphy, pulmonary deposition of ciclesonide delivered via HFA-pMDI was approximately 52% of the inhaled dose, with even lung distribution in healthy individuals and in patients with asthma [4, 17]. In contrast, pulmonary deposition of budesonide delivered via pMDI was approximately 18% [26]. Therefore, this study is consistent with previous trials that report low oropharyngeal deposition of ciclesonide.
This study also indicated that ciclesonide activation to des-CIC in the oropharynx was low (8%). This may be due to low amounts of esterases in the oropharynx that can hydrolyze ciclesonide. No data are currently available to confirm the level of carboxylesterase expression in the oropharynx. The molar dose-adjusted AUC0–60 min of oropharyngeal des-CIC was only 4% of that of budesonide. However, the glucocorticoid receptor binding affinities of des-CIC and budesonide are similar [24]. From a clinical standpoint, the parent compound strategy is a useful means of delivering a potent anti-inflammatory agent to its site of action and potentially reduces the risk of local side effects. Given the results of our study, it can be anticipated that ciclesonide will have a lower incidence of local side effects than budesonide.
An IC may be swallowed and absorbed from the gastrointestinal tract into the systemic circulation and may contribute, especially when administered repeatedly over a prolonged period and/or in high doses, to reduced bone formation [14], cataract development [7], and cortisol suppression [5, 9]. Budesonide has an oral bioavailability of 11%, whereas ciclesonide has a very low oral bioavailability of less than 1%, with almost complete first-pass metabolism [15]. Greater oropharyngeal deposition of budesonide, combined with a correspondingly greater potential for being swallowed and absorbed from the gastrointestinal tract, may explain the higher incidence of systemic side effects with budesonide than with ciclesonide. Consistent with these findings, a recent study revealed that budesonide therapy results in significant cortisol suppression, whereas ciclesonide therapy does not [10]. Further comparative trials are necessary to confirm the improved safety profile of ciclesonide.
In conclusion, this study indicates that oropharyngeal deposition of ciclesonide and des-CIC is less than half that of budesonide. Low oropharyngeal deposition is due to physical properties of ciclesonide such as small particle size. In addition, although similar inhalers were used in this study, differences between the HFA-pMDI and the CFC-pMDI may also contribute to the reduced oropharyngeal deposition of ciclesonide. Furthermore, activation of ciclesonide to des-CIC in the upper oropharynx is low. Reduced deposition and low activation in the oropharynx may explain the low frequency of oropharyngeal side effects demonstrated for ciclesonide in clinical studies [3, 6, 20].
References
(2002) Ciclesonide: BY 9010, ciclesonide-DPI, ciclesonide-MDI, EL 876. Drugs R&D 3:407–410
Barry PW, O’Callaghan C (1996) Inhalational drug delivery from seven different spacer devices. Thorax 51:835–840
Bernstein JA, Noonan MJ, Rim C, Fish J, Kundu S, Williams J et al (2004) Ciclesonide has minimal oropharyngeal side effects in the treatment of patients with moderate-to-severe asthma. J Allergy Clin Immunol 113:S113
Bethke TD, Boudreau RJ, Hasselquist BE, Davidson P, Leach CL, Drollmann A et al (2002) High lung deposition of ciclesonide in 2D- and 3D-imaging. Eur Respir J 20(Suppl 38):109s
Boorsma M, Andersson N, Larsson P, Ullman A (1996) Assessment of the relative systemic potency of inhaled fluticasone and budesonide. Eur Respir J 9:1427–1432
Chapman KR, Patel P, Boulet LP, D’Urzo AD, Alexander M, Mehra S et al (2002) Efficacy and long-term safety of ciclesonide in asthmatic patients as demonstrated in a 52 week long study. Eur Respir J 20(Suppl 38):373s
Cumming RG, Mitchell P, Leeder SR (1997) Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med 337: 8–14
Engelstätter R, Banerji D, Steinijans VW, Wurst W (2004) Low incidence of oropharyngeal adverse events in asthma patients treated with ciclesonide: results from a pooled analysis. Am J Respir Crit Care Med 169:A92
Grahnen A, Jansson B, Brundin RM, Ling-Andersson A, Lonnebo A, Johansson M et al (1997) A dose–response study comparing suppression of plasma cortisol induced by fluticasone propionate from Diskhaler and budesonide from Turbuhaler. Eur J Clin Pharmacol 52:261–267
Hansel T, Engelstätter R, Benezet O, Kafé H, Ponitz HH, Cheung D et al (2003) Once daily ciclesonide (80 μg or 320 μg) is equally effective as budesonide 200 μg given twice daily: a 12-week study in asthma patients. Eur Respir J 22(Suppl 45):410s
Jackson LD, Polygenis D, McIvor RA, Worthington I (1999) Comparative efficacy and safety of inhaled corticosteroids in asthma. Can J Clin Pharmacol 6:26–37
Kelly HW (1999) Comparative potency and clinical efficacy of inhaled corticosteroids. Respir Care Clin N Am 5:537–553
Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ (2002) Lung deposition of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone: a cross-over study in healthy volunteers. Chest 122:510–516
Leech JA, Hodder RV, Ooi DS, Gay J (1993) Effects of short-term inhaled budesonide and beclomethasone dipropionate on serum osteocalcin in premenopausal women. Am Rev Respir Dis 148:113–115
Nave R, Bethke TD, van Marle SP, Zech K (2004) Pharmacokinetics of [14 C]ciclesonide after oral and intravenous administration to healthy subjects. Clin Pharmacokinet 43:479–486
Nave R, Fisher R, Zech K (2003) In vitro metabolism of ciclesonide in the human lung and liver as determined by use of precision-cut tissue slices. Am J Respir Crit Care Med 167(Suppl):A771
Newman S, Salmon A, Nave R, Drollmann A (2004) High lung deposition of 99mTc-labelled ciclesonide administered via HFA-MDI to asthma patients. Eur Respir J 24(suppl 48):583s
Newman SP, Brown J, Steed KP, Reader SJ, Kladders H (1998) Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devices. Chest 113:957–63
Pedersen S, Steffensen G, Ohlsson SV (1993) The influence of orally deposited budesonide on the systemic availability of budesonide after inhalation from a Turbuhaler. Br J Clin Pharmacol 36:211–214
Postma DS, Sevette C, Martinat Y, Schlösser N, Aumann J, Kafé H (2001) Treatment of asthma by the inhaled corticosteroid ciclesonide given either in the morning or evening. Eur Respir J 17:1083–1088
Rohatagi S, Appajosyula S, Derendorf H, Szefler S, Nave R, Zech K et al (2004) Risk–benefit value of inhaled glucocorticoids: a pharmacokinetic/pharmacodynamic perspective. J Clin Pharmacol 44:37–47
Rohatagi S, Arya V, Zech K, Nave R, Hochhaus G, Jensen BK et al (2003) Population pharmacokinetics and pharmacodynamics of ciclesonide. J Clin Pharmacol 43:365–378
Rohatagi S, Derendorf H, Zech K, Nave R, Banerji D (2003) PK/PD of inhaled corticosteroids: the risk/benefit of inhaled ciclesonide. J Allergy Clin Immunol 111:S218
Stoeck M, Riedel R, Hochhaus G, Haefner D, Masso JM, Schmidt B et al (2004) In vitro and in vivo anti-inflammatory activity of the new glucocorticoid ciclesonide. J Pharmacol Exp Ther 309:249–258
Szefler SJ, Herron J, Lloyd M, Rohatagi S, Williams JE, Kundu S et al (2003) High doses of the novel inhaled steroid ciclesonide have no effect on HPA-axis function in patients with moderate-to-severe persistent asthma. J Allergy Clin Immunol 111:S216
Thorsson L, Edsbäcker S, Conradson T-B (1994) Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J 7:1839–1844
Toogood JH, Jennings B, Baskerville J, Anderson J, Johansson SA (1984) Dosing regimen of budesonide and occurrence of oropharyngeal complications. Eur J Respir Dis 65:35–44
Toogood JH, White FA, Baskerville JC, Fraher LJ, Jennings B (1997) Comparison of the antiasthmatic, oropharyngeal, and systemic glucocorticoid effects of budesonide administered through a pressurized aerosol plus spacer or the Turbuhaler dry powder inhaler. J Allergy Clin Immunol 99:186–193
Weinbrenner A, Hüneke D, Zschiesche M, Engel G, Timmer W, Steinijans VW et al (2002) Circadian rhythm of serum cortisol after repeated inhalation of the new topical steroid ciclesonide. J Clin Endocrinol Metab 87:2160–2163
Williamson IJ, Matusiewicz SP, Brown PH, Greening AP, Crompton GK (1995) Frequency of voice problems and cough in patients using pressurized aerosol inhaled steroid preparations. Eur Respir J 8:590–592
Acknowledgements
The following investigators participated in this study: Manfred Hartmann, MD, MSc, Bernhard Hauns, MD, Ulrich Kilian, MD, and Wolfgang Timmer, MD. The authors would like to thank Mr. Werner Meyer (MDS Pharmaservices, Fehraltorf, Switzerland) for performing the bioanalytical work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nave, R., Zech, K. & Bethke, T.D. Lower oropharyngeal deposition of inhaled ciclesonide via hydrofluoroalkane metered-dose inhaler compared with budesonide via chlorofluorocarbon metered-dose inhaler in healthy subjects. Eur J Clin Pharmacol 61, 203–208 (2005). https://doi.org/10.1007/s00228-005-0910-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0910-0