Abstract
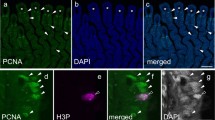
The mantle of the pearl oyster Pinctada fucata was adopted for the proliferation profile study in our work and a proliferation hot spot was found in the outer epithelia of mantle central zone using the BrdU immunohistochemistry method. This result contradicts the previous research that the mantle has numerous growth centers all over the mantle epithelium, with the same proliferation activity throughout the whole mantle outer epithelial cells. This is the first report on the different proliferation features on the whole mantle where Alcian Blue/PAS staining analysis and ultrastructural observation with the aid of transmission electron microscope (TEM) demonstrated distinct features of the epithelium in four different regions of the mantle. Results from the present investigation displayed that in the outer epithelium of the marginal zone in mantle outer fold, organelles such as mitochondria and endoplasmic reticulum (ER) were well-developed and double membrane bounded vesicles were present; in the outer epithelia of mantle central zone, stem-like cells with a high ratio of nucleus to cytoplasm and comparatively undeveloped organelles were detected. Together with the observations of the cell proliferation profile of different regions of the mantle, a hypothetic model for the proliferation and differentiation of the pearl oyster’s mantle is proposed: there exists a proliferation “hot spot” in the outer epithelial cells of central zone and the proliferation ability decreases progressively from this “hot spot” towards the marginal zone; the whole mantle’s differentiation occurs continuously with its growth and the direction is from the proliferation ‘hot spot’ (central zone) towards the marginal zone. Furthermore, another interesting result was found when the proliferation rate was investigated together with the tidal rhythm: the proliferation activity was found to be closely correlated with the tidal rhythm, indicating that the mantle outer epithelia’s proliferation rhythm might be the impetus of the shell’s daily growth bands.







Similar content being viewed by others
References
Abello P, Warman CG, Naylor E (1997) Circatidal moulting rhythm in the shore crab Carcinus maenas. J Mar Biol Assoc UK 77:277–280
Acosta-Salmón H, Martínez-Fernández E, Southgate PC (2004) A new approach to pearl oyster broodstock selection: can saibo donors be used as future broodstock? Aquaculture 231:205–214
Awaji M, Suzuki T (1998) Monolayer formation and DNA synthesis of the outer epithelial cells from pearl oyster mantle in coculture with amebocytes. In Vitro Cell Dev Biol Anim 34:486–91
Beedham GE (1958) Observations on the non-calcareous component of the shell of the Lamellibranchia. Q J Microsc Sci 99:341–357
Berthelin C, Kellner K, Mathieu M (2000) Histological Characterization and glucose incorporation into glycogen of the Pacific Oyster Crassostrea gigas storage cells. Mar Biotechnol 2:136–145
Broom MJ (1978) Growth and spawning in the pectinid Chlamys opercularis in relation to temperature and phytoplankton concentration. Mar Biol 47:277–285
Bubel A (1973) An electron-microscope investigation of the cells lining the outer surface of the mantle in some marine molluscs. Mar Biol 21:245–255
Cajaraville MP, Díez G, Marigómez JA, Angulo E (1990) Responses of basophilic cells of the digestive gland of mussels to petroleum hydrocarbon exposure. Dis Aquat Org 9:221–228
Checa A (2000) A new model for periostracum and shell formation in Unionidae (Bivalvia, Mollusca). Tissue Cell 32:405–416
Checa A (2002) Fabricational morphology of oblique ribs in Bivalves. J Morphol 254:195–209
Clark GR (1968) Mollusc shell: daily growth lines. Science 161:800–802
Clark GR (1975) Periodic growth and biological rhythms in experimentally grown bivalves. In: Rosenberg GD, Runcorn SK (eds) Growth rhythms and the history of the earth’s rotation. Wiley, London, pp 103–117
Denny PC, Chai Y, Klauser DK, Denny PA (1993) Parenchymal cell proliferation and mechanisms for maintenance of granular duct and acinar cell populations in adult male mouse submandibular gland. Anat Rec 235:475–485
Dix T (1972) Histochemistry of mantle and pearl sac secretory cells in Pinctada maxima (Lamellibranchia). Aust J Zool 20:359–368
Ganagarajah M, Saleuddin ASM (1972) Electron histochemistry of the outer mantle epithelium in Helix pomatia during shell regeneration. Proc Malac Soc Lond 40:71–77
Garcia-Gasca A, Ochoa-Baez R, Betancourt M (1994) Microscopic anatomy of the mantle of the pearl oyster Pinctada Mazatlanica (Hanley, 1856). J Shellfish Res 13:85–91
Guo X, Li YL, He X, Li W, Li YH, Zhang LH, Yang RG, Zhao W (2006) Human bone marrow mesenchymal stem cells differentiate toward endothelial lineage in vitro. Chinese J Pathophy 8:1586–1590
Hanselmann R, Smolowitz R (2000) Identification of proliferating cells in hard clams. Biol Bull 199:199–200
Hurley GV, Tremblay MJ, Couturier C (1987) Age estimation of sea scallop larvae (Placopecten magellanicus) from daily growth lines on shells. J Northwest Atl Fish Sci 7:123–129
Icely JD, Nott JA (1992) Digestion absorption: digestive system and associated organs. In: Harrison FW, Humes AG (eds) Microscopic anatomy of invertebrates. Decapod: Crustacea Wiley-Liss, New York, pp 147–201
Joll L (1988) Daily growth rings in juvenile saucer scallops, Amusium balloti (Bernardi). J Shellfish Res 7:73–76
Kawaguti S, Ikemoto N (1962a) Electron microscopy on the mantle of a bivalved gastropod. Biol J Okayama Univ 8:1–20
Kawaguti S, Ikemoto N (1962b) Electron microscopy on the mantle of a bivalve, Fabulina nitidula. Biol J Okayama Univ 8:21–30
Kniprath E (1972) Formation and structure of the periostracum in Lymnaea stagnalis. Calcif Tissue Res 9:260–271
Kniprath E (1978) Growth of the shell-field in Mytilus (Bivalvia). Zool Scr 7:119–120
Leibson NL, Frolova LT (1994) Winter–spring essential reorganization of cell proliferation in the digestive tract epithelia in the mussel Crenomytilus grayanus. Mar Biol 118:471–477
Lobo-da-Cunha A, Kádár E, Santos RS (2006) Histochemical and ultrastructural characterisation of mantle storage cells in the hydrothermal-vent bivalve Bathymodiolus azoricus. Mar Biol 150:253–260
Machii A, Wada KT (1989) Some marine invertebrates tissue culture. In: Mitsuhashi J (ed) Invertebrate cell system applications. CRC, Boca Raton, pp 225–233
Marigómez I, Lekube X, Cancio I (1999) Immunochemical localisation of proliferating cells in mussel digestive gland tissue. Histochem J 31:781–788
Mcquiston RW (1969) Cyclic activity in the digestive diverticula of Lasaea rubra (Montagu) (Bivalvia: Eulamellibranchia). Proc Malacol Soc Lond 38:483–492
Morse MP, Zardus JD (1997) Bivalvia. In: Harrison FW (ed) Microscopic anatomy of invertebrates. Wiley-Liss, New York, pp 7–118
Morton B (1983) Feeding and digestion in bivalvia. In: Saleuddin ASM, Wilbur M (eds) The mollusca. Academic, New York pp 65–147
Mowry RW (1956) Alcian blue techniques for histochemical study and acidic carbohydrates. J Histochem Cytochem 4:407
Naylor E (1996) Crab clockwork: the case for interactive circatidal and circadian oscillators controlling rhythmic locomotor activity of Carcinus maenas. Chronobiol Int 13:153–161
Okudela K, Ito T, Kameda Y, Nakamura N, Kitamura H (1999) Immunohistochemical analysis for cell proliferation-related protein expression in small cell carcinoma of the esophagus; a comparative study with small cell carcinoma of the lung and squamous cell carcinoma of the esophagus. Histol Histopathol 14:479–485
Orton JH, Amirthalingham C, Bull HO (1927) Notes on shell-deposition in oysters. With a note on the chemical composition of “chalky” deposits in shells of O.edulis. J Mar Biol Assoc UK 14:935–954
Pannella G (1975) Palaeontological clocks and the history of the earth’s rotation. In: Rosenberg GD, Runcorn SK (eds) Growth rhythms and the history of the earth’s rotation. Wiley, London, pp 253–284
Parsons GJ, Robinson SMC, Roff JC, Dadswell MJ (1993) Daily growth rates as indicated by valve ridges in postlarval giant scallop (Placopecten magellanicus) (Bivalvia: Pectinidae). Can J Fish Aquat Sci 50:456–464
Pipe RK (1987) Ultrastructural and cytochemical study on interactions between nutrient storage cells and gametogenesis in the mussel Mytilus edulis. Mar Biol 96:519–528
Potswald HE (1977) Further observations on the structure and function of the operculum in Spirorbis moerchi (Serpulidae: Spirorbinae). Biol Bull 152:209–220
Saleuddin ASM (1970) Electron microscopic study of mantle of normal and regenerating Helix. Can J Zool 48:409–416
Salvini-Plawen LV (1988) The structure and function of molluscan digestive systems. In: Trueman ER, Clarke MR (eds) The mollusca. Academic, Orlando pp 301–379
Samuel P, Maroney JR, Barber AA, Wilbur KM (1957) Studies on shell formation VI. The effects of dinitrophenol in mantle respiration, and shell deposition. Biol Bull 112:92–96
Schöne B, Tanabe K, Dettman D, Sato S (2003) Environmental controls on shell growth rates and δ18O of the shallow-marine bivalve mollusk Phacosoma japonicum in Japan. Mar Biol 142:473–485
Simkiss K (1960) Some properties of the organic matrix of the shell of the cockle (Cardium edule) Proc Malac Soc Lond 34:89–95
Thompson I (1975) Biological clocks and shell growth in bivalves. In: Runcorn S (ed) Growth rhythms and the history of the Earth’s rotation. Wiley, London, pp 149–161
Tsujii T (1960) Studies on the mechanisms of shell and pearl formation in Mollusca. J Fac Fish Pref Univ Mie 5:1–70
Vogt G (1994) Life-cycle and functional cytology of the hepatopancreatic cells of Astacus astacus. Crustacea, Decapoda Zoomorphol 144:83–101
Wada K (1964) Studies on the mineralization of the calcified tissue in molluscs. VII. Histological and histochemical studies of organic matrices in shells. Bull Natn Pearl Res Lab 9:1078–1086
Wang RC, Wang SP (1993) Pearl oyster aquaculture and pearl cultivation. Publishing Company of Ocean University of Qingdao, Qingdao, pp 205–243
Westermann B, Schmidtberg H, Beuerlein K (2005) Functional morphology of the mantle of Nautilus pompilius (Mollusca, Cephalopoda). J Morphol 264:277–285
Wilbur KM (1964) Shell formation and regeneration. In: Wilbur KM, Yonge CM (eds) Physiology of mollusca, vol. 1. Academic, New York, pp 243–282
Wilbur KM, Saleuddin ASM (1983) Shell formation. In: Saleuddin ASM, Wilbur KM (eds) The mollusca. Academics, New York, pp 235–287
Yonge CM (1926) The digestive diverticula in Lamellibranchia. Trans R Soc Edinb 54:703–718
Zaldibar B, Cancio I, Marigómez I (2004) Circatidal variation in epithelial cell proliferation in the mussel digestive gland and stomach. Cell Tissue Res 318:395–402
Acknowledgments
This work was financially supported by the National High Technology Research and Development Program of China (2006AA09Z413, 2006AA09Z441, 2006AA10A415), and the National Natural Science Foundation of China (no. 30530600).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Yin.
Rights and permissions
About this article
Cite this article
Fang, Z., Feng, Q., Chi, Y. et al. Investigation of cell proliferation and differentiation in the mantle of Pinctada fucata (Bivalve, Mollusca). Mar Biol 153, 745–754 (2008). https://doi.org/10.1007/s00227-007-0851-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0851-5