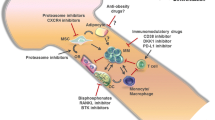
Abstract
Researchers globally are working towards finding a cure for multiple myeloma (MM), a destructive blood cancer diagnosed yearly in ~750,000 people worldwide (Podar et al. in Expert Opin Emerg Drugs 14:99–127, 2009). Although MM targets multiple organ systems, it is the devastating skeletal destruction experienced by over 90 % of patients that often most severely impacts patient morbidity, pain, and quality of life. Preventing bone disease is therefore a priority in MM treatment, and understanding how and why myeloma cells target the bone marrow (BM) is fundamental to this process. This review focuses on a key area of MM research: the contributions of the bone microenvironment to disease origins, progression, and drug resistance. We describe some of the key cell types in the BM niche: osteoclasts, osteoblasts, osteocytes, adipocytes, and mesenchymal stem cells. We then focus on how these key cellular players are, or could be, regulating a range of disease-related processes spanning MM growth, drug resistance, and bone disease (including osteolysis, fracture, and hypercalcemia). We summarize the literature regarding MM-bone cell and MM-adipocyte relationships and subsequent phenotypic changes or adaptations in MM cells, with the aim of providing a deeper understanding of how myeloma cells grow in the skeleton to cause bone destruction. We identify avenues and therapies that intervene in these networks to stop tumor growth and/or induce bone regeneration. Overall, we aim to illustrate how novel therapeutic target molecules, proteins, and cellular mediators may offer new avenues to attack this disease while reviewing currently utilized therapies.




Similar content being viewed by others
References
Podar K, Tai Y-T, Hideshima T et al (2009) Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs 14:99–127
Noopur R, Vescio R, Montgomery CW et al (2015) Bone marker-directed dosing of zoledronic acid for the prevention of skeletal complications in patients with multiple myeloma: results of the Z-MARK study. Clin Cancer Res. doi:10.1158/1078-0432.CCR-15-1864
Reagan MR, Rosen CJ (2015) Navigating the bone marrow niche: translational insights and cancer-driven dysfunction. Nat Rev Rheumatol. doi:10.1038/nrrheum.2015.160
Xiong J, Piemontese M, Onal M et al (2015) Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One 10:e0138189. doi:10.1371/journal.pone.0138189
Kristensen HB, Andersen TL, Marcussen N et al (2014) Osteoblast recruitment routes in human cancellous bone remodeling. Am J Pathol 184:778–789. doi:10.1016/j.ajpath.2013.11.022
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238. doi:10.1002/jbmr.320
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192. doi:10.1038/nm.3074
Delgado-Calle J, Anderson J, Cregor MD et al (2016) Bidirectional Notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res 76:1089–1100. doi:10.1158/0008-5472.CAN-15-1703
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM (2009) Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 19:109–124. doi:10.1016/j.bbi.2008.05.010
Fazeli PK, Horowitz MC, MacDougald OA et al (2013) Marrow fat and bone-new perspectives. J Clin Endocrinol Metab 98:935–945. doi:10.1210/jc.2012-3634
Scheller EL, Rosen CJ (2014) What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci 1311:14–30. doi:10.1111/nyas.12327
Naveiras O, Nardi V, Wenzel PL et al (2009) Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460:259–263. doi:10.1038/nature08099
Cawthorn WP, Scheller EL, Learman BS et al (2014) Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 20:368–375. doi:10.1016/j.cmet.2014.06.003
Zhou BO, Yue R, Murphy MM et al (2014) Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15:154–168. doi:10.1016/j.stem.2014.06.008
Liu Y, Strecker S, Wang L et al (2013) Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One 8:e71318. doi:10.1371/journal.pone.0071318
Chan CKF, Seo EY, Chen JY et al (2015) Identification and specification of the mouse skeletal stem cell. Cell 160:285–298. doi:10.1016/j.cell.2014.12.002
Gao B, Huang Q, Lin Y-S et al (2014) Dose-dependent effect of estrogen suppresses the osteo-adipogenic transdifferentiation of osteoblasts via canonical Wnt signaling pathway. PLoS One 9:e99137. doi:10.1371/journal.pone.0099137
Miyakoshi N, Sato K, Abe T et al (1999) Histomorphometric evaluation of the effects of ovariectomy on bone turnover in rat caudal vertebrae. Calcif Tissue Int 64:318–324
de Paula FJA, de Araújo IM, Carvalho AL et al (2015) The relationship of fat distribution and insulin resistance with lumbar spine bone mass in women. PLoS One 10:e0129764. doi:10.1371/journal.pone.0129764
Bonnet N, Somm E, Rosen CJ (2014) Diet and gene interactions influence the skeletal response to polyunsaturated fatty acids. Bone 68:100–107. doi:10.1016/j.bone.2014.07.024
Doucette CR, Horowitz MC, Berry R et al (2015) A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J Cell Physiol. doi:10.1002/jcp.24954
Colaianni G, Brunetti G, Faienza MF et al (2014) Osteoporosis and obesity: role of Wnt pathway in human and murine models. World J Orthop 5:242–246. doi:10.5312/wjo.v5.i3.242
Lecka-Czernik B, Stechschulte LA (2014) Bone and fat: a relationship of different shades. Arch Biochem Biophys 561:124–129. doi:10.1016/j.abb.2014.06.010
Martin RB, Zissimos SL (1991) Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone 12:123–131
Styner M, Pagnotti GM, Galior K et al (2015) Exercise regulation of marrow fat in the setting of PPARγ agonist treatment in female C57BL/6 mice. Endocrinology 156:2753–2761. doi:10.1210/en.2015-1213
Xuan D, Han Q, Tu Q et al (2016) Epigenetic modulation in periodontitis: interaction of adiponectin and JMJD3-IRF4 axis in macrophages. J Cell Physiol 231:1090–1096. doi:10.1002/jcp.25201
Adler BJ, Kaushansky K, Rubin CT (2014) Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol 10:737–748. doi:10.1038/nrendo.2014.169
Gavin KM, Gutman JA, Kohrt WM et al (2015) De novo generation of adipocytes from circulating progenitor cells in mouse and human adipose tissue. FASEB J. doi:10.1096/fj.15-278994
Shen W, Scherzer R, Gantz M et al (2012) Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab 97:1337–1346. doi:10.1210/jc.2011-2605
Shen W, Velasquez G, Chen J et al (2014) Comparison of the relationship between bone marrow adipose tissue and volumetric bone mineral density in children and adults. J Clin Densitom 17:163–169. doi:10.1016/j.jocd.2013.02.009
Scheller EL, Doucette CR, Learman BS et al (2015) Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun 6:7808. doi:10.1038/ncomms8808
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317. doi:10.1080/14653240600855905
Sivasubramaniyan K, Lehnen D, Ghazanfari R et al (2012) Phenotypic and functional heterogeneity of human bone marrow- and amnion-derived MSC subsets. Ann N Y Acad Sci 1266:94–106. doi:10.1111/j.1749-6632.2012.06551.x
Galli D, Vitale M, Vaccarezza M (2014) Bone marrow-derived mesenchymal cell differentiation toward myogenic lineages: facts and perspectives. Biomed Res Int 2014:762695. doi:10.1155/2014/762695
Moirangthem RD, Singh S, Adsul A et al (2015) Hypoxic niche-mediated regeneration of hematopoiesis in the engraftment window is dominantly affected by oxygen tension in the milieu. Stem Cells Dev 24:2423–2436. doi:10.1089/scd.2015.0112
Qian H, Buza-Vidas N, Hyland CD et al (2007) Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1:671–684. doi:10.1016/j.stem.2007.10.008
Yoshihara H, Arai F, Hosokawa K et al (2007) Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1:685–697. doi:10.1016/j.stem.2007.10.020
Stier S, Ko Y, Forkert R et al (2005) Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med 201:1781–1791. doi:10.1084/jem.20041992
Kunisaki Y, Bruns I, Scheiermann C et al (2013) Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502:637–643. doi:10.1038/nature12612
Abboud C, Lichtman M (2001) Williams’ hematology, 6th edn. McGraw-Hil, New York
Isern J, García-García A, Martín AM et al (2014) The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 3:e03696. doi:10.7554/eLife.03696
Méndez-Ferrer S, Michurina TV, Ferraro F et al (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834. doi:10.1038/nature09262
Seshadri M, Qu C-K (2016) Microenvironmental regulation of hematopoietic stem cells and its implications in leukemogenesis. Curr Opin Hematol. doi:10.1097/MOH.0000000000000251
Kiel MJ, Yilmaz OH, Iwashita T et al (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121. doi:10.1016/j.cell.2005.05.026
Shiozawa Y, Pedersen EA, Havens AM et al (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Investig 121:1298–1312. doi:10.1172/JCI43414
Yu VWC, Scadden DT (2016) Heterogeneity of the bone marrow niche. Curr Opin Hematol 23:331–338. doi:10.1097/MOH.0000000000000265
Oyajobi BO, Franchin G, Williams PJ et al (2003) Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood 102:311–319. doi:10.1182/blood-2002-12-3905
Terpos E, Politou M, Viniou N, Rahemtulla A (2005) Significance of macrophage inflammatory protein-1 alpha (MIP-1alpha) in multiple myeloma. Leuk Lymphoma 46:1699–1707. doi:10.1080/10428190500175049
Hashimoto T, Abe M, Oshima T et al (2004) Ability of myeloma cells to secrete macrophage inflammatory protein (MIP)-1alpha and MIP-1beta correlates with lytic bone lesions in patients with multiple myeloma. Br J Haematol 125:38–41
Croucher PI, McDonald MM, Martin TJ (2016) Bone metastasis: the importance of the neighborhood. Nat Rev Cancer 16:373–386. doi:10.1038/nrc.2016.44
Lawson MA, McDonald MM, Kovacic NN et al (2015) Osteoclasts control re-activation of dormant myeloma cells by remodeling the endosteal niche. Nat Commun 6:8983. doi:10.1038/ncomms9983
Reagan MR, Mishima Y, Glavey SV et al (2014) Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood 124:3250–3259. doi:10.1182/blood-2014-02-558007
Fu R, Liu H, Zhao S et al (2014) Osteoblast inhibition by chemokine cytokine ligand3 in myeloma-induced bone disease. Cancer Cell Int 14:132. doi:10.1186/s12935-014-0132-6
Giuliani N, Ferretti M, Bolzoni M et al (2012) Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia 26:1391–1401. doi:10.1038/leu.2011.381
Delgado-Calle J, Bellido T, Roodman GD (2014) Role of osteocytes in multiple myeloma bone disease. Curr Opin Support Palliat Care 8:407–413. doi:10.1097/SPC.0000000000000090
Habibi H, Abroun S, Hajifathali A et al (2013) Osteogenic inhibition in multiple myeloma. Cell J 15:266–271
Roccaro AM, Sacco A, Purschke WG et al (2014) SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep 9:118–128. doi:10.1016/j.celrep.2014.08.042
Azab AK, Runnels JM, Pitsillides C et al (2009) CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 113:4341–4351. doi:10.1182/blood-2008-10-186668
Takeuchi K, Abe M, Hiasa M et al (2010) Tgf-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth. PLoS One 5:e9870. doi:10.1371/journal.pone.0009870
Li X, Pennisi A, Yaccoby S (2008) Role of decorin in the antimyeloma effects of osteoblasts. Blood 112:159–168. doi:10.1182/blood-2007-11-124164
Krevvata M, Silva BC, Manavalan JS et al (2014) Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood 124:2834–2846. doi:10.1182/blood-2013-07-517219
Chen Z, Orlowski RZ, Wang M et al (2014) Osteoblastic niche supports the growth of quiescent multiple myeloma cells. Blood 123:2204–2208. doi:10.1182/blood-2013-07-517136
Reagan MR, Liaw L, Rosen CJ, Ghobrial IM (2015) Dynamic interplay between bone and multiple myeloma: emerging roles of the osteoblast. Bone 75:161–169. doi:10.1016/j.bone.2015.02.021
Yaccoby S (2010) Osteoblastogenesis and tumor growth in myeloma. Leuk Lymphoma 51:213–220. doi:10.3109/10428190903503438
Yaccoby S, Wezeman MJ, Zangari M et al (2006) Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica 91:192–199
Schmiedel BJ, Scheible CA, Nuebling T et al (2013) RANKL expression, function, and therapeutic targeting in multiple myeloma and chronic lymphocytic leukemia. Cancer Res 73:683–694. doi:10.1158/0008-5472.CAN-12-2280
Eda H, Santo L, Wein MN et al (2016) regulation of sclerostin expression in multiple myeloma by Dkk-1: a potential therapeutic strategy for myeloma bone disease. J Bone Miner Res. doi:10.1002/jbmr.2789
Ng AC, Khosla S, Charatcharoenwitthaya N et al (2011) Bone microstructural changes revealed by high-resolution peripheral quantitative computed tomography imaging and elevated DKK1 and MIP-1α levels in patients with MGUS. Blood 118:6529–6534. doi:10.1182/blood-2011-04-351437
Drake MT (2014) Unveiling skeletal fragility in patients diagnosed with MGUS: no longer a condition of undetermined significance? J Bone Miner Res 29:2529–2533. doi:10.1002/jbmr.2387
Caers J, Deleu S, Belaid Z et al (2007) Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia 21:1580–1584. doi:10.1038/sj.leu.2404658
Liu Z, Xu J, He J et al (2015) Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget 6:34329–34341. doi:10.18632/oncotarget.6020
Medina EA, Oberheu K, Polusani SR et al (2014) PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia. doi:10.1038/leu.2014.112
Hofmann JN, Liao LM, Pollak MN et al (2012) A prospective study of circulating adipokine levels and risk of multiple myeloma. Blood 120:4418–4420. doi:10.1182/blood-2012-06-438606
Hofmann JN, Birmann BM, Teras LR et al (2016) Low levels of circulating adiponectin are associated with multiple myeloma risk in overweight and obese individuals. Cancer Res. doi:10.1158/0008-5472.CAN-15-2406
Fowler JA, Lwin ST, Drake MT et al (2011) Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood 118:5872–5882. doi:10.1182/blood-2011-01-330407
Dalamaga M, Diakopoulos KN, Mantzoros CS (2012) The role of adiponectin in cancer: a review of current evidence. Endocr Rev 33:547–594. doi:10.1210/er.2011-1015
Arita Y, Kihara S, Ouchi N et al (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83. doi:10.1006/bbrc.1999.0255
Hofmann JN, Moore SC, Lim U et al (2013) Body mass index and physical activity at different ages and risk of multiple myeloma in the NIH-AARP diet and health study. Am J Epidemiol 177:776–786. doi:10.1093/aje/kws295
Greenfield DM, Boland E, Ezaydi Y et al (2014) Endocrine, metabolic, nutritional and body composition abnormalities are common in advanced intensively-treated (transplanted) multiple myeloma. Bone Marrow Transplant 49:907–912. doi:10.1038/bmt.2014.63
Abbott MJ, Roth TM, Ho L et al (2015) Negative skeletal effects of locally produced adiponectin. PLoS One 10:e0134290. doi:10.1371/journal.pone.0134290
Hu H, Pu Y, Lu S et al (2015) The osteogenesis effect and underlying mechanisms of local delivery of gAPN in extraction sockets of beagle dogs. Int J Mol Sci 16:24946–24964. doi:10.3390/ijms161024946
Falank C, Fairfield H, Reagan M (2016) Signaling mechanisms between bone marrow adipose tissue and multiple myeloma cells. Front Endocrinol (Lausanne). doi:10.3389/fendo.2016.00067
Fowler JA, Mundy GR, Lwin ST, Edwards CM (2012) Bone marrow stromal cells create a permissive microenvironment for myeloma development: a new stromal role for Wnt inhibitor Dkk1. Cancer Res 72:2183–2189. doi:10.1158/0008-5472.CAN-11-2067
Reagan MR, Ghobrial IM (2012) Multiple myeloma-mesenchymal stem cells: characterization, origin, and tumor-promoting effects. Clin Cancer Res 18:342–349. doi:10.1158/1078-0432.CCR-11-2212
Roccaro AM, Sacco A, Maiso P et al (2013) BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Investig 123:1542–1555. doi:10.1172/JCI66517
Nyangoga H, Mercier P, Libouban H et al (2011) Three-dimensional characterization of the vascular bed in bone metastasis of the rat by microcomputed tomography (MicroCT). PLoS One 6:e17336. doi:10.1371/journal.pone.0017336
Alexandrakis MG, Neonakis IK, Pappa CA et al (2015) Immunohistochemical expression of endoglin offers a reliable estimation of bone marrow neoangiogenesis in multiple myeloma. J Cancer Res Clin Oncol 141:1503–1509. doi:10.1007/s00432-015-1952-z
Moschetta M, Mishima Y, Kawano Y et al (2016) Targeting vasculogenesis to prevent progression in multiple myeloma. Leukemia. doi:10.1038/leu.2016.3
Giuliani N, Morandi F, Tagliaferri S et al (2007) The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 110:334–338. doi:10.1182/blood-2006-11-059188
Avilés A, Neri N, Huerta-Guzmán J, Nambo MJ (2013) Randomized clinical trial of zoledronic acid in multiple myeloma patients undergoing high-dose chemotherapy and stem-cell transplantation. Curr Oncol 20:e13–e20. doi:10.3747/co.20.1055
Morgan GJ, Davies FE, Gregory WM et al (2013) Long-term follow-up of MRC Myeloma IX trial: survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res 19:6030–6038. doi:10.1158/1078-0432.CCR-12-3211
Tibullo D, Di Rosa M, Giallongo C et al (2015) Bortezomib modulates CHIT1 and YKL40 in monocyte-derived osteoclast and in myeloma cells. Front Pharmacol 6:226. doi:10.3389/fphar.2015.00226
Yang Y, Blair HC, Shapiro IM, Wang B (2015) The proteasome inhibitor carfilzomib suppresses parathyroid hormone-induced osteoclastogenesis through a RANKL-mediated signaling pathway. J Biol Chem 290:16918–16928. doi:10.1074/jbc.M115.663963
Hoy SM (2016) Carfilzomib triple combination therapy: a review in relapsed multiple myeloma. Target Oncol 11:255–262. doi:10.1007/s11523-016-0428-7
Muz B, Ghazarian RN, Ou M et al (2016) Spotlight on ixazomib: potential in the treatment of multiple myeloma. Drug Des Dev Ther 10:217–226. doi:10.2147/DDDT.S93602
Anderson G, Gries M, Kurihara N et al (2006) Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood 107:3098–3105. doi:10.1182/blood-2005-08-3450
San Miguel J, Weisel K, Moreau P et al (2013) Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 14:1055–1066. doi:10.1016/S1470-2045(13)70380-2
Bolomsky A, Schreder M, Meißner T et al (2014) Immunomodulatory drugs thalidomide and lenalidomide affect osteoblast differentiation of human bone marrow stromal cells in vitro. Exp Hematol 42:516–525. doi:10.1016/j.exphem.2014.03.005
Munemasa S, Sakai A, Kuroda Y et al (2008) Osteoprogenitor differentiation is not affected by immunomodulatory thalidomide analogs but is promoted by low bortezomib concentration, while both agents suppress osteoclast differentiation. Int J Oncol 33:129–136
Raje N, Vadhan-Raj S, Willenbacher W et al (2016) Evaluating results from the multiple myeloma patient subset treated with denosumab or zoledronic acid in a randomized phase 3 trial. Blood Cancer J 6:e378. doi:10.1038/bcj.2015.96
Terpos E, Confavreux CB, Clézardin P (2015) Bone antiresorptive agents in the treatment of bone metastases associated with solid tumours or multiple myeloma. Bonekey Rep 4:744. doi:10.1038/bonekey.2015.113
Vallet S, Mukherjee S, Vaghela N et al (2010) Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci USA 107:5124–5129. doi:10.1073/pnas.0911929107
Terpos E, Kastritis E, Christoulas D et al (2012) Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann Oncol 23:2681–2686. doi:10.1093/annonc/mds068
Chantry AD, Heath D, Mulivor AW et al (2010) Inhibiting activin-A signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. J Bone Miner Res 25:2633–2646. doi:10.1002/jbmr.142
Vallet S, Raje N (2011) Bone anabolic agents for the treatment of multiple myeloma. Cancer Microenviron Off J Int Cancer Microenviron Soc 4:339–349. doi:10.1007/s12307-011-0090-7
Iyer SP, Beck JT, Stewart AK et al (2014) A Phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br J Haematol 167:366–375. doi:10.1111/bjh.13056
Hu B, Chen Y, Usmani SZ et al (2013) Characterization of the molecular mechanism of the bone-anabolic activity of carfilzomib in multiple myeloma. PLoS One 8:e74191. doi:10.1371/journal.pone.0074191
Croucher PI, De Hendrik R, Perry MJ et al (2003) Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res 18:482–492. doi:10.1359/jbmr.2003.18.3.482
Vanderkerken K, De Leenheer E, Shipman C et al (2003) Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res 63:287–289
Coleman R, Powles T, Paterson A et al (2015) Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386:1353–1361. doi:10.1016/S0140-6736(15)60908-4
Smith MR, Saad F, Coleman R et al (2012) Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet (Lond Engl) 379:39–46. doi:10.1016/S0140-6736(11)61226-9
Yaccoby S, Ling W, Zhan F et al (2007) Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood 109:2106–2111. doi:10.1182/blood-2006-09-047712
Pozzi S, Fulciniti M, Yan H et al (2013) In vivo and in vitro effects of a novel anti-Dkk1 neutralizing antibody in multiple myeloma. Bone 53:487–496. doi:10.1016/j.bone.2013.01.012
Fulciniti M, Tassone P, Hideshima T et al (2009) Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114:371–379. doi:10.1182/blood-2008-11-191577
Heath DJ, Chantry AD, Buckle CH et al (2009) Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res 24:425–436. doi:10.1359/jbmr.081104
Styner M, Thompson WR, Galior K et al (2014) Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 64:39–46. doi:10.1016/j.bone.2014.03.044
Acknowledgments
The authors thank Dr. Michael Erard, Scientific Editor and Writing consultant at Maine Medical Center Research Institute (MMCRI) for editorial assistance and Dr. Clifford Rosen (MMCRI) for his expertise in Marrow Adipose. Dr. Reagan’s lab is supported by MMCRI Start-up funds, a pilot project grant from NIH/NIGMS (P30GM106391), and the NIH/NIDDK (R24 DK092759-01). Dr. Michelle McDonald is supported by The Kay Stubbs Cancer Council NSW Project Grant RG 16-03.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Michelle McDonald, Heather Fairfield, Carolyne Falank, Michaela R. Reagan are no potential conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
McDonald, M.M., Fairfield, H., Falank, C. et al. Adipose, Bone, and Myeloma: Contributions from the Microenvironment. Calcif Tissue Int 100, 433–448 (2017). https://doi.org/10.1007/s00223-016-0162-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0162-2