Abstract
The tumor necrosis factor-related cytokine receptor activator of nuclear factor kappa B ligand (RANKL) has been proposed as predictor of incident type 2 diabetes mellitus, and experimental blockade of RANKL resulted in a marked improvement of glucose tolerance. Denosumab is a fully human monoclonal antibody that binds to RANKL and prevents osteoclast formation, function and survival, leading to fracture risk reduction. The aim of our study was to investigate glucometabolic parameters, insulin resistance, and lipid profile in non-diabetic women receiving denosumab. Forty-eight women with postmenopausal osteoporosis were enrolled and treated with a subcutaneous dose (60 mg) of denosumab. At baseline and after 4, 12, ad 24 weeks, insulin resistance was computed by homeostasis model assessment of insulin resistance (HOMA-IR) and total cholesterol, triglycerides and HDL cholesterol were also measured. At baseline and after 24 weeks, bone turn-over markers were also evaluated. After denosumab administration, with the exception of a slight reduction of insulin and HOMA-IR values after 4 weeks (p < 0.05), neither fasting plasma glucose nor insulin and insulin resistance were significantly changed. Lipid parameters remained unchanged at each time-points of this study. A reduction of C-telopeptide of type 1 collagen (−63 %, p < 0.0001) and osteocalcin (−45 %, p < 0.0001), as bone resorption and formation markers, respectively, were observed after 24 weeks. Baseline levels of bone biomarkers were not predictive of HOMA-IR, and changes of osteocalcin were not associated to markers of glucose control. In osteoporotic otherwise healthy postmenopausal women, denosumab was not associated with relevant modification of insulin resistance and lipid profile.
Similar content being viewed by others
Introduction
The tumor necrosis factor (TNF)-related cytokine receptor activator of nuclear factor kappa B ligand (RANKL) is a key cytokine inducing differentiation of hematopoietic precursors common to the monocyte/macrophage and osteoclast lineages into multinucleated, bone-resorbing cells. RANKL binding to its cognate receptor RANK further promotes formation, activation, and survival of osteoclasts. Its role in bone metabolism is highlighted from both animal and human observations, showing that deleting or inactivating mutations of RANKL and RANK genes result in the absence of osteoclasts and osteopetrosis [1, 2]. The characterization of the RANK, RANKL, and its soluble decoy receptor osteoprotegerin (OPG) has led to the development of denosumab, a fully human IgG2 monoclonal antibody to RANKL, which is currently used as an antiresorptive treatment for postmenopausal osteoporosis. By binding RANKL, denosumab prevents the interaction of RANKL with its receptor RANK, on osteoclasts and osteoclast precursors, and reversibly inhibits osteoclast-mediated bone resorption. Denosumab administration was associated with a reduction in the risk of vertebral, non-vertebral, and hip fractures in women with osteoporosis [3, 4]. It is known that RANK and RANKL are both expressed in liver and pancreatic β-cells, and activation of the transcription factor NF-kB and downstream inflammatory signaling pathways are involved in hepatic insulin resistance and β-cell dysfunction [5]. In particular, RANKL levels have been associated to many human diseases as osteometabolic, cardiovascular or inflammatory bowel diseases, rheumatoid arthritis, and multiple myeloma [6–9]. Moreover, OPG levels, as a surrogate marker of the overall activity of this cytokine network, are elevated in subjects with type 2 diabetes mellitus (DM), suggesting a possible pathophysiological role of this system [10]. In the prospective population-based Bruneck Study, a high serum concentration of soluble RANKL emerged as an independent risk predictor of type 2 DM manifestation, and experimental blockade of RANKL in mouse models of type 2 DM resulted in a marked improvement of glucose tolerance [11]. To date, limited data exist regarding the glucometabolic effects derived by blocking RANKL in humans.
Osteocalcin (BGP), an osteoblast derived non-collagenous protein in bone, has been recently reported to promote insulin secretion by pancreatic beta-cells and insulin sensitivity in peripheral tissues [12]; because denosumab, as well as other antiresorptive treatments, may reduce BGP levels, it is possible that insulin secretion may be consequently impaired.
Thus, the main aim of this study was to determine whether and to what extent denosumab can modify glucose control. The secondary aim was to assess whether such a treatment affects other major cardiovascular risk factors including plasma concentrations of total cholesterol, high-density lipoprotein (HDL) and LDL cholesterol, and triglycerides.
Materials and Methods
Our prospective study included a group of Caucasian postmenopausal women, referring to the Center for Osteoporosis of the Department of Clinical and Experimental Medicine, University Hospital of Messina (Messina, Italy) from November 2012 to April 2013. Women were eligible for inclusion if they had a bone mineral density T score of −2.5 SD or less at lumbar spine or femoral neck and at least one morphometric vertebral fracture in accordance to Genant’s classification. Secondary causes of osteoporosis were previously excluded, and patients with low (<75 nmol/L) serum 25(OH)D were not considered. Patients were also excluded if they were affected by chronic renal or liver failure, heart failure, hypo/hypercalcemia, prior diagnosis of cancer or diabetes mellitus, or if they had received medical treatment with corticosteroids in the last 6 months. They all had never received before an antiosteoporotic medical treatment, with the exception of vitamin D, and were at the first administration of denosumab. Recruited women whose therapy was modified during the follow-up period and women absent for surveys were removed from the final analysis. At baseline, we collected data on dietary calcium intake by a validated food frequency questionnaire [13] and measured body mass index (BMI) and waist to hip circumferences ratio (WHR). BMI was calculated as weight divided by height squared. Waist and hip circumferences were measured by a plastic tape meter at the level of the umbilicus and the greater trochanters. Recruited subjects were advised not to modify their diet and lifestyle during the observation period; they were also supplemented with oral cholecalciferol (25,000 IU/monthly) in order to maintain their vitamin D status. The study was performed in accordance with the ethical standards of our institutional research committee and with the 1964 Declaration of Helsinki and its later amendments; written informed consent was obtained from all individual participants included in this study. Serum samples were collected after 12- to 14-h fast, at baseline, and at 4, 12 and 24 weeks after 60 mg of denosumab subcutaneous administration. At each time-point, fasting plasma glucose and insulin were measured; insulin resistance was computed by homeostasis model assessment of insulin resistance (HOMA-IR) with the formula: fasting plasma glucose (mmol/L) times fasting serum insulin (pmol/L) divided by 135. The reliability of HOMA-IR has been validated in normoglycemic subjects against insulin sensitivity measured directly from the euglycemic-hyperinsulinemic clamp technique [14]. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides were measured by routine enzymatic methods, and LDL-C was calculated in accordance to Friedewald’s formula: total cholesterol (mmol/L) - (HDL cholesterol (mmol/L) + triglycerides (mmol/L)/2.2). At baseline and at the end of the study levels of BGP, as a marker of bone formation, and serum C-telopeptide of type 1 collagen (CTX), as a marker of bone resorption, 25(OH)D, PTH, Insulin-Like Growth Factor 1 (IGF-1), and HbA1c were recorded. Serum calcium, phosphorus, and creatinine were measured using standard laboratory techniques, and glucose was measured using the glucose oxidase–peroxidase method (normal laboratory reference range, 65–110 mg/dL); concentrations of CTX were assessed by immunoenzymatic assay, levels of IGF-1 by RIA, HbA1c, PTH, and 25(OH)D were detected by high-performance liquid chromatography. Immunoenzymatic assays were used to measure serum BGP (Invitrogen Ltd, UK) with an intra-assay coefficient of variance (CV) of 3.1 %, an inte-rassay CV of 3.5 %, a detection limit of 0.08 µg/L, insulin levels (Beckman Coulter, USA) with intra and inter-assay CVs <4.2 and <5.6 %, respectively, and a lowest detectable level of 0.21 pmol/L.
Statistical analyses were performed using MedCalc software (version 10.2.0.0; MedCalc Software, Mariakerke, Belgium). Values were expressed as mean ± SD. Biochemical data were compared by repeated measures analysis of variances, paired t test, or Wilcoxon matched rank-sum test for paired data as appropriate. All reported p values were two-sided. Pearson’s correlation coefficient was used to analyze the degree of association between two variables. A multiple regression analysis was performed to determine the influence of one independent variable after correcting for the others. For all the tests, a p value of 0.05 or less was considered to indicate statistical significance. A post hoc power analysis was performed with G-power software [15].
Results
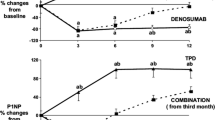
Overall, a total of forty-eight Caucasian postmenopausal women were enrolled in this study, and their clinical characteristics are shown in Table 1. Three patients were excluded from the final analyses: one patient was excluded because she started antihypertensive treatment with ramipril and another one because she received treatment with dexamethasone for acute bronchitis during the observation period; the third patient was excluded because she moved to other city and missed the subsequent monitoring after baseline. At baseline, and at the end of the study, 25(OH)D levels were negatively associated to BMI and total cholesterol (p < 0.05). Age, BMI, WHR, T score values, BGP, and CTX serum levels were not associated to HOMA-IR, HbA1c, and lipid measurements. After denosumab administration, with the exception of a slight, but significant, reduction of insulin and HOMA-IR values after 4 weeks, no significant changes were observed in glucometabolic and lipid parameters at each time-points of this study (Table 2); in addition, BMI and WHR were not significantly modified at the end of the study, in comparison to baseline. As expected, after 24 weeks, a reduction of CTX (0.79 ± 0.33 vs. 0.29 ± 0.11 ng/mL at baseline and after 24 weeks, respectively, p < 0.0001) and BGP (24.21 ± 3.72 vs. 13.12 ± 2.42 µg/L, at baseline and after 24 weeks, respectively, p < 0.0001) was observed. IGF-1 (127.14 ± 28.1 vs. 125.8 ± 24.9 nmol/L, at baseline and after 24 weeks, respectively), PTH (22.82 ± 4.61 vs. 23.21 ± 3.82 ng/L, at baseline and after 24 weeks, respectively), and 25(OH)D (36.48 ± 5.51 vs. 35.76 ± 4.12 nmol/L, at baseline and after 24 weeks, respectively) were not significantly changed at the end of this study. After multiple regression analysis, baseline levels of bone biomarkers were not predictive of HOMA-IR, and changes of BGP were not associated to any metabolic modification. A post hoc sample size calculation indicated that a much larger cohort of patients would be needed to reach the statistical power to confirm the metabolic effects of denosumab in our population study.
Discussion
Our study examined the effects of a single dose of denosumab on glucose metabolism and insulin resistance in postmenopausal women with osteoporosis. Previously, we had reported the effects of estrogen and raloxifene, a selective estrogen receptor modulator, on glucose tolerance and plasma lipid concentration [16, 17]. Although epidemiologic and experimental data support a role for the RANK/RANKL/OPG system in the pathogenesis of type 2 DM, our findings show that denosumab mediated blockade of RANKL in non-diabetic postmenopausal women may have not relevant clinical effects on fasting glucose and HOMA-IR.
In an Italian prospective population-based study, over a follow-up period of 15 years, subjects who were diagnosed as affected by type 2 DM showed, at baseline, higher serum concentrations of soluble RANKL in comparison to subjects without incident diabetes [11]; thus, RANKL was highlighted as a potential risk factor for type 2 DM. In accordance with this observation in humans, it was previously reported that hepatocyte-specific RANK knockout (RANKLKO) mice did not develop insulin resistance after high-fat diet in comparison with the wild-type counterpart (RANKWT); moreover, leptin deficient mouse (ob/ob), which represent a model of insulin resistance and hyperglycemia, when crossbreed with RANKLKO mice, showed significantly lower fasting glucose levels and HOMA-IR [11]. Tamura et al. reported that a moderate reduction of NF-κB activity in the liver is enough to improve glucose tolerance caused by reduced expression of gluconeogenesis genes, such as those encoding phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, in addition to reduced expression of gene encoding peroxisome proliferator-activated receptor gamma coactivator-1α [18].
In a prior post hoc analysis of the FREEDOM trial, a 3-year, randomized, double-blind, placebo-controlled study that enrolled 7808 postmenopausal women with osteoporosis, denosumab had no effect on incident diabetes or fasting serum glucose in women without diabetes at baseline [19]. At a further post hoc analysis of the FREEDOM trial, denosumab did not appear to affect glucose levels in women with diabetes or prediabetes; however, it was reported a fasting serum glucose lowering with denosumab in women with diabetes who were not currently using any antidiabetic medication [20]. Except for a slight, but significant reduction of HOMA-IR after 4 weeks, we did not observe any effects of denosumab on insulin resistance during our study. Our results are substantially consistent with a very recent study of Passeri et al. [21], who studied the effects of denosumab on hepatic insulin resistance. In accordance to FREEDOM data [3], we observed no effect on fasting serum glucose and; moreover, the treatment with denosumab did not change lipid profile over the 24 weeks observation period. The observed transient and mild change in insulin resistance at 4 weeks is not known if it has a clinical significance and should be confirmed in a wider population.
Interestingly, as would be expected, we observed a significant reduction of BGP at the end of our study. This is a common finding under antiresorptive treatment for osteoporosis and may have implication on glucose tolerance [22, 23]. It has been consistently documented that diabetic patients have lower circulating BGP levels in comparison to healthy people [24, 25]; thus, modifications of circulating BGP levels could impair glucose metabolism also in subjects without diabetes. It is known that antiresorptive drugs in postmenopausal women reduce not only bone resorption but also bone formation markers including BGP. However, data from large randomized placebo-controlled trials of alendronate, zoledronic acid, and denosumab did not show an increased risk of weight gain, glucose intolerance, or diabetes in comparison with the placebo arms [19]. In particular, in the FREEDOM trial, the bone formation marker procollagen type I N-terminal peptide was significantly reduced since the first month, and its reduction was maintained before the administration of the following dose [3, 19]. This is consistent with the observed significant reduction of BGP in our study detected after 6 months. However, effects on uncarboxilated BGP, the active form of BGP, have only been investigated for alendronate, and decreased values were reported with treatment, already after 3 months [22]. Although we did not dose uncarboxilated BGP, we suppose that denosumab, which reduces total BGP, could also reduce the levels of the uncarboxilated form. Taken together the direct effects on RANKL blockade and the indirect effects on BGP, it is likely that glucose metabolism may not be relevantly changed by denosumab treatment.
Furthermore, 25(OH)D was associated to total cholesterol levels at baseline, but we did not observe significant change of any lipid parameters all over the study period although vitamin D supplementation, and this is consistent with our recent findings in atorvastatin-treated postmenopausal women taking vitamin D [26].
Finally, we recognize that limitations of the present study are the small sample size and the absence of a placebo group which does weaken its conclusions, but certainly does not invalidate the findings. We acknowledged also that HOMA-IR has been used as a surrogate marker, hyperinsulinemic clamp being the gold standard to assess insulin sensitivity; moreover, we studied the effects of a single dose of denosumab over time and considered a group of only postmenopausal women excluding men; thus, we do not know what could occur with successive doses over a longer observation time and whether these results may be intended generalizable to men. At the same time, the strength of this investigation lies in the homogeneous population considered, consisting of all postmenopausal women without relevant comorbidities.
Since the large prevalence of insulin resistance, further studies, investigating different doses of denosumab and involving men and possibly also subjects with metabolic syndrome and diabetes, are needed.
References
Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der HeidenA Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13:1015–1024
Wada T, Nakashima T, Oliveira-dos-Santos AJ, Gasser J, Hara H, Schett G, Penninger JM (2005) The molecular scaffold Gab2 is acrucial component of RANK signaling and osteoclastogenesis. Nat Med 11:394–399
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, Trial FREEDOM (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11(2):183–190
Kiechl S, Schett G, Schwaiger J, Seppi K, Eder P, Egger G, Santer P, Mayr A, Xu Q, Willeit J (2007) Soluble receptor activator of nuclear factor-kappa B ligand and risk for cardiovascular disease. Circulation 116(4):385–391
Schett G, Kiechl S, Redlich K, Oberhollenzer F, Weger S, Egger G, Mayr A, Jocher J, Xu Q, Pietschmann P, Teitelbaum S, Smolen J, Willeit J (2004) Soluble RANKL and risk of non traumatic fracture. JAMA 291(9):1108–1113
Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, Viniou N, Yataganas X, Goldman JM, Rahemtulla A (2003) Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102(3):1064–1069
Ziolkowska M, Kurowska M, Radzikowska A, Luszczykiewicz G, Wiland P, Dziewczopolski W, Filipowicz-Sosnowska A, Pazdur J, Szechinski J, Kowalczewski J, Rell-Bakalarska M, Maslinski W (2002) High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor alpha treatment. Arthritis Rheum 46(7):1744–1753
Moschen AR, Kaser A, Enrich B, Ludwiczek O, Gabriel M, Obrist P, Wolf AM, Tilg H (2005) The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 54(4):479–487
Nabipour I, Kalantarhormozi M, Larijani B, Assadi M, Sanjdideh Z (2010) Osteoprotegerin in relation to type 2 diabetes mellitus and the metabolic syndrome in postmenopausal women. Metabolism 59(5):742–747
Kiechl S, Wittmann J, Giaccari A, Knoflach M, Willeit P, Bozec A, Moschen AR, Muscogiuri G, Sorice GP, Kireva T, Summerer M, Wirtz S, Luther J, Mielenz D, Billmeier U, Egger G, Mayr A, Oberhollenzer F, Kronenberg F, Orthofer M, Penninger JM, Meigs JB, BonoraE Tilg H, Willeit J, Schett G (2013) Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med 19(3):358–363
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Montomoli M, Gonnelli S, Giacchi M, Mattei R, Cuda C, Rossi S, Gennari C (2002) Validation of a food frequency questionnaire for nutritional calcium intake assessment in Italian women. Eur J Clin Nutr 56(1):21–30
Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M (2000) Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 23:57–63
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Lasco A, Gaudio A, Morabito N, Previti M, Mileto A, Frisina N, Cucinotta D (2004) Effects of a long-term treatment with raloxifene on insulin sensitivity in postmenopausal women. Diabetologia 47(3):571–574
Lasco A, Alvaro S, Frisina N, Di Benedetto A, Denuzzo G, Cucinotta D (2000) Long-term transdermal estrogen therapy improves lipid profile but not insulin resistance in healthy postmenopausal women. Diabetes Care 23(3):422–424
Tamura Y, Ogihara T, Uchida T, Ikeda F, Kumashiro N, Nomiyama T, Sato F, Hirose T, Tanaka Y, Mochizuki H, Kawamori R, Watada H (2007) Amelioration of glucose tolerance by hepatic inhibition of nuclear factor kappaB indb/db mice. Diabetologia 50(1):131–141
Schwartz AV, Schafer AL, Grey A, Vittinghoff E, Palermo L, Lui LY, Wallace RB, Cummings SR, Black DM, Bauer DC, Reid IR (2013) Effects of antiresorptive therapies on glucose metabolism: results from the FIT, HORIZON-PFT, and FREEDOM trials. J Bone Miner Res 28(6):1348–1354
Napoli N, Vittinghoff E, Pannacciulli N, Crittenden D, Yun J, Wang A, Wagman R, Schwartz A (2014) Effect of Denosumab on Fasting Glucose Concentrations in Postmenopausal Women with Osteoporosis: Results From Subjects With Diabetes or Prediabetes From the FREEDOM Trial. J Bone Miner Res 29(Suppl 1):S36 1104 (Oral Presentation)
Passeri E, Benedini S, Costa E, Corbetta S (2015) A Single 60 mg Dose of Denosumab Might Improve Hepatic Insulin Sensitivity in Postmenopausal Non diabetic Severe Osteoporotic Women. Int J Endocrinol 2015:352858. doi:10.1155/2015/352858
Schafer AL, Sellmeyer DE, Schwartz AV, Rosen CJ, Vittinghoff E, Palermo L, Bilezikian JP, Shoback DM, Black DM (2011) Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH Study). J Clin Endocrinol Metab 96:1982–1989
Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B (2009) Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 94:827–832
Catalano A, Morabito N, Di Vieste G, Pintaudi B, Cucinotta D, Lasco A, Di Benedetto A (2013) Phalangeal quantitative ultrasound and metabolic control in pre-menopausal women with type 1 diabetes mellitus. J Endocrinol Invest 36(5):347–351
Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, Sugimoto T (2009) Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab 94:45–49
Catalano A, Morabito N, Basile G, Cucinotta D, Lasco A (2015) Calcifediol improves lipid profile in osteopenic atorvastatin-treated postmenopausal women. Eur J Clin Invest 45(2):144–149
Author Contribution
Lasco Antonino and Catalano Antonino designed this study and prepared the first draft of this paper. Catalano Antonino is the guarantor. Lasco Antonino, Morabito Nunziata, Basile Giorgio, Atteritano Marco, Gaudio Agostino, Giorgianni Grazia Maria, Morini Elisabetta, Faraci Bianca, Bellone Federica, and Catalano Antonino contributed to the experimental work. Lasco Antonino and Catalano Antonino were responsible for statistical analysis of the data. All authors revised the paper critically for intellectual content and approved the final version. All the authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of this paper are investigated and properly resolved.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lasco Antonino, Morabito Nunziata, Basile Giorgio, Atteritano Marco, Gaudio Agostino, Giorgianni Grazia Maria, Morini Elisabetta, Faraci Bianca, Bellone Federica, and Catalano Antonino declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Rights and permissions
About this article
Cite this article
Lasco, A., Morabito, N., Basile, G. et al. Denosumab Inhibition of RANKL and Insulin Resistance in Postmenopausal Women with Osteoporosis. Calcif Tissue Int 98, 123–128 (2016). https://doi.org/10.1007/s00223-015-0075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0075-5