Abstract
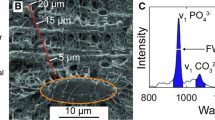
Using an ovariectomized (OVX) ovine model, we provide an analysis of the timing of changes in bone following estrogen deficiency. The expression of genes known to regulate osteoclastogenesis, matrix production, and mineralization, as measured by real-time RT-PCR, was significantly increased by 12 months; and increased expression was maintained through to 31 months post-OVX compared to controls. FTIR spectroscopy confirmed that mineralized crystals were less mature than in controls 12 months post-OVX and were even less so by 31 months. The mineral-to-matrix ratio was significantly reduced by 31 months, while the ratio of mature to immature collagen cross-linking was initially increased at 12 months and subsequently reduced at 31 months post-OVX. In contrast, trabecular number, thickness, and separation were unchanged at 12 months. Significant reductions in trabecular number and thickness and a significant increase in trabecular separation were observed 31 months after OVX. Most notably perhaps these combined changes led to a significant reduction in the compressive strength of trabecular bone after 31 months. The results indicate that there is an initial increase in bone turnover, which is accompanied by a change in bone composition. This is followed by a continued increase in bone resorption and relative reduction in bone formation, leading to deterioration in bone microarchitecture. Ultimately, these cumulative changes led to a significant reduction in the compressive strength of bones following 31 months of estrogen deficiency. These findings provide important insight into the time sequence of changes during osteoporosis.







Similar content being viewed by others
References
Avioli LV (1991) Heterogeneity of osteoporotic syndromes and the response to calcitonin therapy. Calcif Tissue Int 49(2):16–19
Parkinson IH, Forbes D, Sutton-Smith P, Fazzalari NL (2010) Model-independent 3D descriptors of vertebral cancellous bone architecture. J Osteoporos 2010:641578
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90(12):6508–6515
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89(2):309–319
McNamara LM, Ederveen AGH, Lyons CG, Price C, Schaffler MB, Weinans H, Prendergast PJ (2006) Strength of cancellous bone trabecular tissue from normal, ovariectomized and drug-treated rats over the course of ageing. Bone 39(2):392–400
Brennan O, Kennedy OD, Lee TC, Rackard SM, O’Brien FJ (2009) Biomechanical properties across trabeculae from the proximal femur of normal and ovariectomised sheep. J Biomech 42(4):498–503
Brennan O, Kennedy OD, Lee TC, Rackard SM, O’Brien FJ, McNamara LM (2011) The effects of estrogen deficiency and bisphosphonate treatment on tissue mineralisation and stiffness in an ovine model of osteoporosis. J Biomech 44(3):386–390
Brennan M, Gleeson JP, Browne M, O’Brien FJ, Thurner PJ, McNamara LM (2011) Site specific increase in heterogeneity of trabecular bone tissue mineral during estrogen deficiency. Eur Cell Mater 15(21):396–406
Bailey AJ, Sims TJ, Ebbesen EN, Mansell JP, Thomsen JS, Mosekilde L (1999) Age-related changes in the biochemical properties of human cancellous bone collagen: relationship to bone strength. Calcif Tissue Int 65(3):203–210
Bailey AJ, Wotton SF, Sims TJ, Thompson PW (1992) Post-translational modifications in the collagen of human osteoporotic femoral head. Biochem Biophys Res Commun 185(3):801–805
Tran BN, Nguyen ND, Center JR, Eisman JA, Nguyen TV (2009) Enhancement of absolute fracture risk prognosis with genetic marker: the collagen I alpha 1 gene. Calcif Tissue Int 85(5):379–388
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21(2):195–214
Boskey AL (1995) Osteopontin and related phosphorylated sialoproteins: effects on mineralization. Ann N Y Acad Sci 760:249–256
Hunter GK, Goldberg HA (1993) Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci USA 90(18):8562–8565
Tye CE, Rattray KR, Warner KJ, Gordon JA, Sodek J, Hunter GK, Goldberg HA (2003) Delineation of the hydroxyapatite-nucleating domains of bone sialoprotein. J Biol Chem 278(10):7949–7955
Tye CE, Hunter GK, Goldberg HA (2005) Identification of the type I collagen-binding domain of bone sialoprotein and characterization of the mechanism of interaction. J Biol Chem 280(14):13487–13492
Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GA, Stucky GD, Morse DE, Hansma PK (2005) Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater 4(8):612–616
Grynpas MD, Tupy JH, Sodek J (1994) The distribution of soluble, mineral-bound, and matrix-bound proteins in osteoporotic and normal bones. Bone 15(5):505–513
Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC (1998) Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest 101(9):1942–1950
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25(7):1468–1486
Keaveny TM, Borchers RE, Gibson LJ, Hayes WC (1993) Trabecular bone modulus and strength can depend on specimen geometry. J Biomech 26:991–1000
Un K, Bevill G, Keaveny TM (2006) The effects of side-artifacts on the elastic modulus of trabecular bone. J Biomech 39(11):1955–1963
Pacifici R (1996) Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 11(8):1043–1051
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS (1994) Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331(16):1056–1061
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T (2001) Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 28(1):80–86
Turner CH, Forwood MR, Rho JY, Yoshikawa T (1994) Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res 9(1):87–97
Wronski TJ, Dann LM, Scott KS, Cintron M (1989) Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int 45(6):360–366
D’Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G (2008) Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 43(1):92–100
Khosla S (2001) The OPG/RANKL/RANK system. Endocrinology 142(12):5050–5055
Smith-Mungo LI, Kagan HM (1998) Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol 16(7):387–398
Bailey AJ (2002) Changes in bone collagen with age and disease. J Musculoskelet Neuronal Interact 2(6):529–531
Han S, McBride DJ, Losert W, Leikin S (2008) Segregation of type I collagen homo- and heterotrimers in fibrils. J Mol Biol 383(1):122–132
Truong LH, Kuliwaba JS, Tsangari H, Fazzalari NL (2006) Differential gene expression of bone anabolic factors and trabecular bone architectural changes in the proximal femoral shaft of primary hip osteoarthritis patients. Arthritis Res Ther 8(6):R188
Couchourel D, Aubry I, Delalandre A, Lavigne M, Martel-Pelletier J, Pelletier JP, Lajeunesse D (2009) Altered mineralization of human osteoarthritic osteoblasts is attributable to abnormal type I collagen production. Arthritis Rheum 60(5):1438–1450
Cheng Z, Yao W, Zimmermann EA, Busse C, Ritchie RO, Lane NE (2009) Prolonged treatments with antiresorptive agents and PTH have different effects on bone strength and the degree of mineralization in old estrogen-deficient osteoporotic rats. J Bone Miner Res 24(2):209–220
Gadeleta SJ, Boskey AL, Paschalis E, Carlson C, Menschik F, Baldini T, Peterson M, Rimnac CM (2000) A physical, chemical, and mechanical study of lumbar vertebrae from normal, ovariectomized, and nandrolone decanoate-treated cynomolgus monkeys (Macaca fascicularis). Bone 27(4):541–550
Busse B, Hahn M, Soltau M, Zustin J, Puschel K, Duda GN, Amling M (2009) Increased calcium content and inhomogeneity of mineralization render bone toughness in osteoporosis: mineralization, morphology and biomechanics of human single trabeculae. Bone 45(6):1034–1043
Ciarelli TE, Fyhrie DP, Parfitt AM (2003) Effects of vertebral bone fragility and bone formation rate on the mineralization levels of cancellous bone from white females. Bone 32(3):311–315
Kennedy OD, Brennan O, Rackard SM, Staines A, O’Brien FJ, Taylor D, Lee TC (2009) Effects of ovariectomy on bone turnover, porosity, and biomechanical properties in ovine compact bone 12 months postsurgery. J Orthop Res 27(3):303–309
Healy C, Kennedy OD, Brennan O, Rackard SM, O’Brien FJ, Lee TC (2010) Structural adaptation and intracortical bone turnover in an ovine model of osteoporosis. J Orthop Res 28(2):248–251
McKee MD, Nanci A (1996) Osteopontin: an interfacial extracellular matrix protein in mineralized tissues. Connect Tissue Res 35(1–4):197–205
Chang IC, Chiang TI, Yeh KT, Lee H, Cheng YW (2010) Increased serum osteopontin is a risk factor for osteoporosis in menopausal women. Osteoporos Int 21(8):1401–1409
Young MF, Kerr JM, Ibaraki K, Heegaard AM, Robey PG (1992) Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clin Orthop Relat Res 281(281):275–294
Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA (1996) Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J 317(pt 1):59–64
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382(6590):448–452
Acknowledgement
The authors acknowledge Dr. Gerald Atkins for helpful discussion and Ms. Lena Truong, Ms. Katie Reeve-Arnold, and Ms. Laura Corrigan for technical assistance. Funding for this study was granted by the Health Research Board under grant numbers RP/2004/229 and RP/2007/179 and by the Higher Education Authority in Ireland under PRTLI Cycle III.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Brennan, O., Kuliwaba, J.S., Lee, T.C. et al. Temporal Changes in Bone Composition, Architecture, and Strength Following Estrogen Deficiency in Osteoporosis. Calcif Tissue Int 91, 440–449 (2012). https://doi.org/10.1007/s00223-012-9657-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-012-9657-7