Abstract
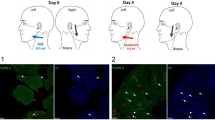
We determined the role of persistent monoarthritis of temporomandibular joint region (TMJ) on bilateral masseter muscle (MM) nociception in male rats using orofacial nocifensive behaviors, phosphorylated extracellular signal-regulated kinase and Fos induction at the trigeminal subnucleus caudalis/upper cervical spinal cord (Vc/C2) region in response to formalin injection to the MM region. TMJ inflammation was induced by local injection of CFA into the left TMJ region. Orofacial nocifensive behaviors evoked by formalin injection ipsilateral or contralateral to the TMJ inflammation appeared to be increased at 1–14 days or at 1, 10 and 14 days after induction of TMJ inflammation, respectively, while increases in behavioral duration were seen mainly in the late phase rather than the early phase. The number of pERK positive cells was investigated in superficial laminae at the Vc/C2 region at 3, 10, 20, 60 and 80 min after MM stimulation with formalin at 14 days after TMJ inflammation. TMJ-inflamed rats displayed greater responses of pERK expression by the ipsilateral MM stimulation at 3–60 min, while contralateral MM stimulation increased pERK expression at 3, 10 and 20 min compared to non-CFA rats. Fos expression by MM stimulation was increased at 14 days after induction of TMJ inflammation regardless of the affected side. These findings showed that persistent TMJ inflammation for 10 and 14 days is sufficient to enhance MM nociception indicated by behaviors and neural responses in superficial laminae at the Vc/C2 region.




Similar content being viewed by others
References
Adwanikar H, Karim F, Gereau RWT (2004) Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain 111:125–135
Aloisi AM, Porro CA, Cavazzuti M, Baraldi P, Carli G (1993) ‘Mirror pain’ in the formalin test: behavioral and 2-deoxyglucose studies. Pain 55:267–273
Ambalavanar R, Moutanni A, Dessem D (2006) Inflammation of craniofacial muscle induces widespread mechanical allodynia. Neurosci Lett 399:249–254
Bereiter DA, Bereiter DF (2000) Morphine and NMDA receptor antagonism reduce c-fos expression in spinal trigeminal nucleus produced by acute injury to the TMJ region. Pain 85:65–77
Bereiter DA, Okamoto K, Bereiter DF (2005) Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain 117:58–67
Cao Y, Li K, Fu KY, Xie QF, Chiang CY, Sessle BJ (2013) Central sensitization and MAPKs are involved in occlusal interference-induced facial pain in rats. J Pain 14:793–807
Chai B, Guo W, Wei F, Dubner R, Ren K (2012) Trigeminal-rostral ventromedial medulla circuitry is involved in orofacial hyperalgesia contralateral to tissue injury. Mol Pain 8:78
Cheng CF, Cheng JK, Chen CY, Rau RH, Chang YC, Tsaur ML (2015) Nerve growth factor-induced synapse-like structures in contralateral sensory ganglia contribute to chronic mirror-image pain. Pain 156:2295–2309
Clavelou P, Pajot J, Dallel R, Raboisson P (1989) Application of the formalin test to the study of orofacial pain in the rat. Neurosci Lett 103:349–353
Cruz CD, Neto FL, Castro-Lopes J, McMahon SB, Cruz F (2005) Inhibition of ERK phosphorylation decreases nociceptive behaviour in monoarthritic rats. Pain 116:411–419
Danziger N, Weil-Fugazza J, LeBars D, Bouhassira D (1999) Alteration of descending modulation of nociception during the course of monoarthritis. J Neurosci 19:2394–2400
Donaldson LF (1999) Unilateral arthritis: contralateral effects. Trends Neurosci 22:495–496
Dubner R (1991) Basic mechanisms of pain associated with deep tissues. Can J Physiol Pharmacol 69:607–609
Fitzgerald M (1983) Influences of contralateral nerve and skin stimulation on neurones in the substantia gelatinosa of the rat spinal cord. Neurosci Lett 36:139–143
Gao YJ, Ji RR (2009) c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J 2:11–17
Hathaway CB, Hu JW, Bereiter DA (1995) Distribution of Fos-like immunoreactivity in the caudal brainstem of the rat following noxious chemical stimulation of the temporomandibular joint. J Comp Neurol 356:444–456
Hu JW, Sessle BJ, Raboisson P, Dallel R, Woda A (1992) Stimulation of craniofacial muscle afferents induces prolonged facilitatory effects in trigeminal nociceptive brain-stem neurones. Pain 48:53–60
Huang WJ, Wang BR, Yao LB et al (2000) Activity of p44/42 MAP kinase in the caudal subnucleus of trigeminal spinal nucleus is increased following perioral noxious stimulation in the mouse. Brain Res 861:181–185
Hubbard RD, Winkelstein BA (2005) Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine (Phila Pa 1976) 30:1924–1932
Hubbard RD, Chen Z, Winkelstein BA (2008) Transient cervical nerve root compression modulates pain: load thresholds for allodynia and sustained changes in spinal neuropeptide expression. J Biomech 41:677–685
Imbe H, Iwata K, Zhou QQ, Zou S, Dubner R, Ren K (2001) Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Implications for persistent temporomandibular pain. Cells Tissues Organs 169:238–247
Iwata K, Tashiro A, Tsuboi Y et al (1999) Medullary dorsal horn neuronal activity in rats with persistent temporomandibular joint and perioral inflammation. J Neurophysiol 82:1244–1253
Ji RR, Baba H, Brenner GJ, Woolf CJ (1999) Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 2:1114–1119
Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ (2002) p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36:57–68
Ji RR, Gereau RW, Malcangio M, Strichartz GR (2009) MAP kinase and pain. Brain Res Rev 60:135–148
Koltzenburg M, Wall PD, McMahon SB (1999) Does the right side know what the left side is doing? Trends Neurosci 22:122–127
Levine JD, Dardick SJ, Basbaum AI, Scipio E (1985) Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J Neurosci 5:1380–1386
Mense S (1993) Nociception from skeletal muscle in relation to clinical muscle pain. Pain 54:241–289
Millan MJ (1999) The induction of pain: an integrative review. Prog Neurobiol 57:1–164
Molander C, Xu Q, Rivero-Melian C, Grant G (1989) Cytoarchitectonic organization of the spinal cord in the rat: II. The cervical and upper thoracic cord. J Comp Neurol 289:375–385
Neto FL, Schradrack J, Ableitner A, Castro-Lopes JM, Bartenstein P, Zieglgansberger W, Tolle TR (1999) Supraspinal metabolic activity changes in the rat during adjuvant monoarthritis. Neuroscience 94:607–621
Noma N, Tsuboi Y, Kondo M et al (2008) Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J Comp Neurol 507:1428–1440
Obata K, Noguchi K (2004) MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci 74:2643–2653
Oh SH, Imbe H, Iwai-Liao Y (2006) TMJ inflammation increases Fos expression in the nucleus raphe magnus induced by subsequent formalin injection of the masseter or hindpaw of rats. Okajimas Folia Anat Jpn 83:43–52
Okamoto K, Imbe H, Tashiro A, Kumabe S, Senba E (2004) Blockade of peripheral 5HT3 receptor attenuates the formalin-induced nocifensive behavior in persistent temporomandibular joint inflammation of rat. Neurosci Lett 367:259–263
Okamoto K, Imbe H, Tashiro A, Kimura A, Donishi T, Tamai Y, Senba E (2005) The role of peripheral 5HT2A and 5HT1A receptors on the orofacial formalin test in rats with persistent temporomandibular joint inflammation. Neuroscience 130:465–474
Okamoto K, Kimura A, Donishi T et al (2006) Persistent monoarthritis of the temporomandibular joint region enhances nocifensive behavior and lumbar spinal Fos expression after noxious stimulation to the hindpaw in rats. Exp Brain Res 170:358–367
Okamoto K, Imbe H, Kimura A, Donishi T, Tamai Y, Senba E (2007) Activation of central 5HT2A receptors reduces the craniofacial nociception of rats. Neuroscience 147:1090–1102
Ren K, Dubner R (2002) Descending modulation in persistent pain: an update. Pain 100:1–6
Sarlani E, Greenspan JD (2003) Evidence for generalized hyperalgesia in temporomandibular disorders patients. Pain 102:221–226
Schaible HG, Grubb BD (1993) Afferent and spinal mechanisms of joint pain. Pain 55:5–54
Segond von Banchet G, Petrow PK, Brauer R, Schaible H-G (2000) Monoarticular antigen-induced arthritis leads to pronounced bilateral upregulation of the expression of neurokinin 1 and bradykinin 2 receptors in dorsal root ganglion neurons of rats. Arthritis Res 2:424–427
Seino D, Tokunaga A, Tachibana T et al (2006) The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain 123:193–203
Shenker N, Haigh R, Roberts E, Mapp P, Harris N, Blake D (2003) A review of contralateral responses to a unilateral inflammatory lesion. Rheumatology 42:1279–1286
Simonic-Kocijan S, Zhao X, Liu W, Wu Y, Uhac I, Wang K (2013) TRPV1 channel-mediated bilateral allodynia induced by unilateral masseter muscle inflammation in rats. Mol Pain 9:68
Suzuki I, Harada T, Asano M et al (2007) Phosphorylation of ERK in trigeminal spinal nucleus neurons following passive jaw movement in rats with chronic temporomandibular joint inflammation. J Orofac Pain 21:225–231
Takeshita S, Hirata H, Bereiter DA (2001) Intensity coding by TMJ-responsive neurons in superficial laminae of caudal medullary dorsal horn of the rat. J Neurophysiol 86:2393–2404
Tashiro A, Okamoto K, Bereiter DA (2009) Chronic inflammation and estradiol interact through MAPK activation to affect TMJ nociceptive processing by trigeminal caudalis neurons. Neuroscience 164:1813–1820
Turp JC, Kowalski CJ, O’Leary N, Stohler CS (1998) Pain maps from facial pain patients indicate a broad pain geography. J Dent Res 77:1465–1472
Wall PD, Woolf CJ (1984) Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol 356:443–458
Woolf CJ, Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288:1765–1768
Worsley MA, Allen CE, Billinton A, King AE, Boissonade FM (2014) Chronic tooth pulp inflammation induces persistent expression of phosphorylated ERK (pERK) and phosphorylated p38 (pp38) in trigeminal subnucleus caudalis. Neuroscience 269:318–330
Wright EF (2000) Referred craniofacial pain patterns in patients with temporomandibular disorder. J Am Dent Assoc 131:1307–1315
Yang CS, Jung CY, Ju JS, Lee MK, Ahn DK (2005) Intracisternal administration of mitogen-activated protein kinase inhibitors reduced IL-1beta-induced mirror-image mechanical allodynia in the orofacial area of rats. Neurosci Lett 387:32–37
Zhou Q, Imbe H, Dubner R, Ren K (1999) Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: implications for persistent orofacial pain. J Comp Neurol 412:276–291
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110
Acknowledgements
This work was partly supported by the MEXT/JSPS KAKENHI Grant Number 16K11679.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kurose, M., Imbe, H., Nakatani, Y. et al. Bilateral increases in ERK activation at the spinomedullary junction region by acute masseter muscle injury during temporomandibular joint inflammation in the rats. Exp Brain Res 235, 913–921 (2017). https://doi.org/10.1007/s00221-016-4852-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4852-9