Abstract
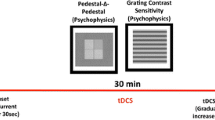
Blindsight has been widely investigated and its properties documented. One property still debated and contested is the puzzling absence of phenomenal visual percepts of visual stimuli that can be detected with perfect accuracy. We investigated the possibility that phenomenal visual percepts of exogenous visual stimuli in patient GY might be induced by using transcranial direct current stimulation. High contrast and low contrast stimuli were presented as a moving grating in his blind hemifield. When left area MT/V5 was anodally stimulated during the presentation of high-contrast gratings, he never reported a phenomenal percept of a moving grating but showed perfect blindsight performance. When applied along with low contrast gratings, for which accuracy was titrated to 60–70 %, performance did not improve but responses were significantly faster. Cathodal stimulation had no effect. Results are explained in the framework of GY’s reorganized cortical connexions and oscillatory patterns known to be involved in awareness in GY. The apparent presence of phenomenal visual percepts in earlier studies is shown to be a semantic confusion about what he means when he says that he sees in his blind field.



Similar content being viewed by others
References
Azzopardi P, Cowey A (2001) Motion discrimination in cortically blind patients. Brain 124:30–46
Barbur JL, Watson JD, Frachowiak RSJ, Zeki S (1993) Conscious visual perception without V1. Brain 116:1293–1302
Blumberger DM, Tran LC, Fitzgerald PB, Hoy KE, Daskalakis ZJ (2012) A randomized double-blind sham-controlled study of transcranial direct current stimulation for treatment-resistant major depression. Front Psychiatry 3:74
Boros K, Poreisz C, Münchau A, Paulus W, Nitsche MA (2008) Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci 27:1292–1300
Bridge H, Thomas O, Jbabdi S, Cowey A (2008) Changes in connectivity after visual cortical brain damage underlie altered visual function. Brain 131:1433–1444
Cowey A (2010) The blindsight saga. Exp Brain Res 200:3–23
Cowey A, Alexander I (2012) Are hemianopic monkeys and a human hemianope aware of visual events in the blind field? Exp Brain Res 219:47–57
Cowey A, Walsh V (2000) Magnetically induced phosphenes in sighted, blind and blindsighted observers. NeuroReport 11:3269–3273
Feurra M, Bianco G, Polizzotto NR, Innocenti I, Rossi A, Rossi S (2011) Cortico-cortical connectivity between right parietal and bilateral primary motor cortices during imagined and observed actions: a combined TMS/tDCS study. Front Neural Circuits 5:10
Ioannides AA, Poghosyan V, Liu L, Saridis GA, Tamietto M, Op de Beeck M, De Tiège X, Weiskrantz L, de Gelder B (2012) Spatiotemporal profiles of visual processing with and without primary visual cortex. Neuroimage 63:1464–1477
Lavidor M, Walsh V (2004) The nature of foveal representation. Nat Rev Neurosci 5:729–735
Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W (2003) Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 114:2220–2222
Pascual-Leone A, Walsh V (2001) Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science 292:510–512
Peña-Gómez C, Sala-Lonch R, Junqué C, Clemente IC, Vidal D, Bargalló N, Falcón C, Valls-Solé J, Pascual-Leone A, Bartrés-Faz D (2011) Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stim. doi:10.1016/j.brs.2011.08.006
Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, Pascual-Leone A, Bikson M (2011) Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stim. doi:10.1016/j.brs.2011.10.001
Riddoch G (1917) Dissociation of visual perceptions due to occipital injuries, with especial reference to appreciation of movement. Brain 40:15–57
Schurger A, Cowey A, Tallon-Baudry C (2006) Induced gamma-band oscillations correlate with awareness in hemianopic patient GY. Neuropsychologia 44:1796–1803
Schurger A, Cowey A, Cohen J, Treisman A, Tallon-Baudry C (2008) Distinct and independent correlates of attention and awareness in a hemianopic patient. Neuropsychologia 46:2189–2197
Silvanto J, Cowey A, Lavie N, Walsh V (2007) Making the blindsighted see. Neuropsychologia 45:3346–3350
Silvanto J, Walsh V, Cowey A (2009) Abnormal functional connectivity between ipsilesional V5/MT+ and contralesional striate cortex (V1) in blindsight. Exp Brain Res 193:645–650
Stoerig P, Barth E (2001) Low-level conscious vision despite unilateral destruction of primary visual cortex. Conscious Cogn 4:574–587
Tamietto M, Pullens P, de Gelder B, Weiskrantz L, Goebel R (2012) Subcortical connections to human amygdala and their changes following destruction of visual cortex. Curr Biol. doi:10.1016/j.cub.2012.06.006
Weiskrantz L, Warrington EK, Sanders MD, Marshall J (1974) Visual capacity in the hemianopic field following a restricted cortical ablation. Brain 97:709–728
Wu D, Qian L, Zorowitz RD, Zhang L, Qu Y, Yuan Y (2012) Effects on decreasing upper-limb post-stroke muscle tone using transcranial direct current stimulation: a Randomized Sham-Controlled Study. Arch Phys Med Rehabil. doi:10.1016/j.apmr.2012.07.022
Zeki S, Ffytche DH (1998) The Riddoch syndrome: insights into the neurobiology of conscious vision. Brain 121:25–45
Acknowledgments
AC was supported by a Leverhulme Emeritus Fellowship and IA by a grant from the EPA Cephalosporin Research Fund, Oxford. AE was supported by a grant from the Dr. Hadwen Trust for Humane Research. We thank subject GY for his continuing interest in blindsight and his willingness to describe his experiences and submit to lengthy psychophysical testing while being stimulated with tDCS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cowey, A., Alexander, I. & Ellison, A. Modulation of cortical excitability can speed up blindsight but not improve it. Exp Brain Res 224, 469–475 (2013). https://doi.org/10.1007/s00221-012-3327-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3327-x