Abstract
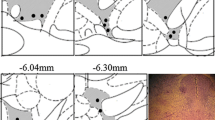
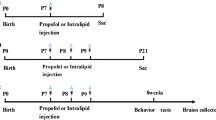
We studied Fos expression in the central nociceptive pathways at different sedative levels in order to clarify the central mechanism of propofol’s nociceptive action. Sprague–Dawley rats received propofol (PRO) or pentobarbital (PEN) and were divided into two groups with different doses of drug administration (light and deep sedative levels) based on the electroencephalogram analysis. Rats at each sedative level received heat stimulation to their face and Fos immunohistochemistry was performed at various brain sites. We also infused lidocaine into the jugular vein to test whether PRO directly activated nociceptors distributed in the vein. Fos expression in two major ascending pain pathways (lateral and medial systems) and descending modulatory system were precisely analyzed following intravenous (i.v.) administration of PRO or PEN. Many Fos protein-like immunoreactive (Fos protein-LI) cells were expressed in the trigeminal spinal nucleus caudalis (Vc), parabrachial nucleus, parafascicular nucleus, a wide area of the primary somatosensory cortex, anterior cingulate cortex, amygdala, periaqueductal gray, solitary tract nucleus, and lateral hypothalamus following heating of the face during PRO or PEN infusion. The number of Fos protein-LI cells was significantly greater in many Central nervous system regions during PRO infusion compared with PEN. Fos expression was significantly greater in the Vc and Periaqueductal gray following greater amount of PRO infusions compared, whereas they were significantly smaller in the Vc in the rats with PEN infusion. The Fos expression was significantly depressed following i.v. infusion of lidocaine before PRO administration. The present findings suggest that PRO is involved in the enhancement of Vc activity through direct activation of the primary afferent fibers innervating veins, resulting in pain induction during infusion.





Similar content being viewed by others
Abbreviations
- ACC:
-
Anterior cingulate cortex
- Aq:
-
Aqueduct
- BLA:
-
Basolateral amygdaroid nucleus
- CNS:
-
Central nervous system
- CeA:
-
Central nucleus of amygdala
- EEG:
-
Electroencephalograph
- HP:
-
Hypothalamus
- INS:
-
Insular
- LPBN:
-
Lateral parabrachial nucleus
- Me5:
-
Mesencephalic nucleus
- PAG:
-
Periaqueductal gray
- PEN:
-
Pentobarbital
- PF:
-
Parafascicular thalamic nucleus
- PRO:
-
Propofol
- Py:
-
Pyramis
- RVM:
-
Rostral ventromedial medulla
- SI:
-
Primary somatosensory cortex
- SC:
-
Superior colliculus
- SN:
-
Substantia nigra
- Sm:
-
Thalamic nucleus submedius
- STN:
-
Solitary tract nucleus
- V3:
-
3rd ventricle
- VBc:
-
Ventrobasal thalamic complex
- Vc:
-
Trigeminal spinal nucleus caudalis
- VLO:
-
Ventrolateral orbital cortex
- cc:
-
Central canal
- scp:
-
Superior cerebellar peduncle
References
Anker-Moller E, Spangsberg N, Arendt-Nielsen L, Schultz P, Kristensen MS, Bjerring P (1991) Subhypnotic doses of thiopentone and propofol cause analgesia to experimentally induced acute pain. Br J Anaesth 66:185–188
Bereiter DA, Bereiter DF (1996) N-Methyl-D-Aspartate and Non-N- Methyl-D-Aspartate receptor antagonism reduces Fos-like immunoreactivity in central trigeminal neurons after corneal stimulation in the rat. Neuroscience 73:249–258
Bodo M, Perjes G, Kalman E, Bacskai E, Berko K, Sarkadi A, Nagy I, Keim KL, Matysik FM, Csomor K, McCarron R, Zagvazdin Y, Rosenthal M, Morrissette C, Herendy E, Szporny L, Nagy Z (2001) Screening for cerebroprotective agents using an in vivo model of cerebral reversible depolarization in awake rats. Pharmacol Res 44:419–429
Bullitt E (1991) Somatotopy of spinal nociceptive processing. J Comp Neurol 312:279–290
Canavero S, Bonicalzi V, Pagni CA, Castellano G, Merante R, Gentile S, Bradac GB, Bergui M, Benna P, Vighetti S (1995) Propofol analgesia in central pain: preliminary clinical observations. J Neurol 242:561–567
Doenicke AW, Roizen MF, Rau J, Kellermann W, Babl J (1996) Reducing pain during propofol injection: the role of the solvent. Anesth Analg 82:472–474
Dong XP, Xu TL (2002) The actions of propofol on gamma-aminobutyric acid-A and glycine receptors in acutely dissociated spinal dorsal horn neurons of the rat. Anesth Analg 95:907–914
Dzoljic E, van Leeuwen R, de Vries R, Dzoljic MR (1997) Vigilance and EEG power in rats: effects of potent inhibitors of the neuronal nitric oxide synthase. Naunyn Schmiedebergs Arch Pharmacol 356:56–61
Ewen A, Archer DP, Samanani N, Roth SH (1995) Hyperalgesia during sedation: effects of barbiturates and propofol in the rat. Can J Anaesth 42:532–540
Grasshoff C, Antkowiak B (2004) Propofol and sevoflurane depress spinal neurons in vitro via different molecular targets. Anesthesiology 101:1167–1176
Hara M, Kai Y, Ikemoto Y (1994) Enhancement by propofol of the gamma-aminobutyric acidA response in dissociated hippocampal pyramidal neurons of the rat. Anesthesiology 81:988–994
Hathaway CB, Hu JW, Bereiter D (1995) Distribution of Fos-like immunoreactivity in the caudal brainstem of the rat following noxious chemical stimulation of the temporomandibular joint. J Comp Neurol 356:444–456
Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkuhler J (2004) Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol 560:249–266
Hu JW, Sessle BJ (1984) Comparison of responses of cutaneous nociceptive and nonnociceptive brain stem neurons in trigeminal subnucleus caudalis (medullary dorsal horn) and subnucleus oralis to natural and electrical stimulation of tooth pulp. J Neurophysiol 52:39–53
Hunt SP, Pini A, Even G (1987) Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328:632–634
Ichinose F, Miyazaki M, Goto T, Takahashi H, Terui K, Niimi Y, Uezono S, Morita S, Yanagida H (1999) Electroencephalographic responses to the formalin test in rats. Pain 80:251–256
Imbe H, Dubner R, Ren K (1999) Masseteric inflammation-induced Fos protein expression in the trigeminal interpolaris/caudalis transition zone: contribution of somatosensory-vagal-adrenal integration. Brain Res 845:165–175
Jansson A, Olin K, Yoshitake T, Hagman B, Herrington MK, Kehr J, Permert J (2004) Effects of isoflurane on prefrontal acetylcholine release and hypothalamic Fos response in young adult and aged rats. Exp Neurol 190:535–543
Jewett BA, Gibbs LM, Tarasiuk A, Kendig JJ (1992) Propofol and barbiturate depression of spinal nociceptive neurotransmission. Anesthesiology 77:1148–1154
Kay B, Rolly G (1977) I.C.I. 35868, a new intravenous induction agent. Acta Anaesthesiol Belg 28:303–316
Kushikata T, Hirota K, Kotani N, Yoshida H, Kudo M, Matsuki A (2005) Isoflurane increases norepinephrine release in the rat preoptic area and the posterior hypothalamus in vivo and in vitro: relevance to thermoregulation during anesthesia. Neuroscience 131:79–86
Lin Q, Peng Y, Wills WD (1994) Glycine and GABAA antagonists reduse the inhibition of primate spinothalamic tract neurons produced by stimulation in periaqueductal gray. Brain Res 654:286–302
Lu H, Xu TL (2002) The general anesthetic pentobarbital slows desensitization and deactivation of the glycine receptor in the rat spinal dorsal horn neurons. J Biol Chem 277:41369–41378
MacIver MB, Mandema JW, Stanski DR, Bland BH (1996) Thiopental uncouples hippocampal and cortical synchronized electroencephalographic activity. Anesthesiology 84:1411–1424
McCulloch MJ, Less NW (1985) Assessment and modification of pain on induction with propofol (Diprivan). Anaesthegia 40:1117–1120
Naguib M, Schmid PG III, Baker MT (2003) The electroencephalographic effects of IV anesthetic doses of melatonin: comparative studies with thiopental and propofol. Anesth Analg 97:238–243
Nicol ME, Moriarty J, Edwards J, Robbie DS, A’Hern RP (1991) Modification of pain on injection of propofol—a comparison between lignocaineand procaine. Anaesthesia. 46:67–69
Nomura H, Ogawa A, Tashiro A, Morimoto T, Hu JW, Iwata K (2002) Induction of Fos protein-like immunoreactivity in the trigeminal spinal nucleus caudalis and upper cervical cord following noxious and non-noxious mechanical stimulation of the whisker pad of the rat with an inferior alveolar nerve transection. Pain 95:225–238
Novikova NS, Kazakova TB, Rogers V, Korneva EA (2004) Expression of the c-fos gene in spinal cord and brain cells in rats subjected to stress in conditions of exposure to various types of halothane anesthesia. Neurosci Behav Physiol 34:407–412
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. 4th edn. Academic Press, San Diego, CA
Petersen-Felix S, Arendt-Nielsen L, Bak P, Fischer M, Zbinden AM (1996) Psychophysical and electrophysiological responses to experimental pain may be influenced by sedation: comparison of the effects of a hypnotic (propofol) and an analgesic (alfentanil). Br J Anaesth 77:165–171
Reichling DB, Basbaum AI (1990) Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: I. GABA-immunoreactive projection neurons in the periaqueductal gray and nucleus raphe magnus. J Comp Neurol 302:370–377
Sandkuhler J, Willmann E, Fu QG (1989) Blockade of GABAA receptors in the midbrain periaqueductal gray abolishes nociceptive spinal dorsal horn neuronal activity. Eur J Pharmacol 160:163–166
Sanna E, Garau F, Harris RA (1995) Novel properties of homomeric beta 1 gamma-aminobutyric acid type A receptors: actions of the anesthetics propofol and pentobarbital. Mol Pharmacol 47:213–217
Senba E, Umemoto S, Kawai Y, Noguchi K (1994) Differential expression of fos family and jun family mRNAs in the rat hypothalamo-pituitary-adrenal axis after immobilization stress. Brain Res Mol Brain Res 24:283–294
Steinbach JH, Akk G (2001) Modulation of GABA(A) receptor channel gating by pentobarbital. J Physiol 537:715–733
Strassman AM, Vos BP, Mineta Y, Naderi S, Borsook D, Burstein R (1993) Fos-like immunoreactivity in the superficial medullary dorsal horn induced by noxious and innocuous thermal stimulation of facial skin in the rat. J Neurophysiol 70:1811–1821
Takemura M, Shimada T, Shigenaga Y (2000) GABA(A) receptor-mediated effects on expression of c-Fos in rat trigeminal nucleus following high- and low-intensity afferent stimulation. Neuroscience 98:325–332
Tan PP, Shyr MH, Yang CH, Kuo TB, Pan WH, Chan SH (1993) Power spectral analysis of the electroencephalographic and hemodynamic correlates of propofol anesthesia in the rat: intravenous infusion. Neurosci Lett 160:205–208
Uchida H, Kishikawa K, Collins JG (1995) Effect of propofol on spinal dorsal horn neurons. Comparison with lack of ketamine effects. Anesthesiology 83:1312–1322
Vijn PC, Sneyd JR (1998) i.v. anaesthesia and EEG burst suppression in rats: bolus injections and closed-loop infusions. Br J Anaesth 81:415–421
Wilder-Smith OH, Kolletzki M, Wilder-Smith CH (1995) Sedation with intravenous infusions of propofol or thiopentone. Effects on pain perception. Anaesthesia 50:218–222
Zhou Q, Imbe H, Dubner R, Ren K (1999) Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: implications for persistent orofacial pain. J Comp Neurol 412:276–291
Zimmerman M (1983) Ethical guidelines for investigation of experimental pain in conscious animals. Pain 16:109–110
Acknowledgments
We thank Profs. R. Dubner and B. J. Sessle for invaluable comments on the manuscript. This study was supported in part by Research Grants from Sato and Uemura Funds from Nihon University School of Dentistry, and a grant from the Dental Research Center, Nihon University School of Dentistry; Nihon University multidisciplinary research grant for KI and Individual Research Grant for YT; a grant from the Ministry of Education, Culture, Sports, Science, and Technology to promote multidisciplinary research projects; a grant from the Ministry of Education, Culture, Sports, Science, and Technology to promote multidisciplinary research projects “Brain Mechanisms for Cognition, Memory and Behavior” at Nihon University. We thank Dr. D. A. Thomas for correcting English usage in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubota, I., Tsuboi, Y., Shoda, E. et al. Modulation of neuronal activity in CNS pain pathways following propofol administration in rats: Fos and EEG analysis. Exp Brain Res 179, 181–190 (2007). https://doi.org/10.1007/s00221-006-0779-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0779-x