Abstract
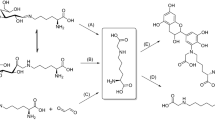
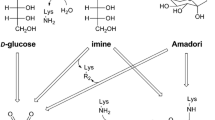
To investigate the influence of high hydrostatic pressure on the reactions of glyoxal with lysine residues, the formation of end-products, namely N-ε-(carboxymethyl)lysine (CML) and 1,3-bis(5-amino-5-carboxypentyl)imidazolium salt (GOLD), was studied in model systems via LC–MS. Following a kinetic interpretation, reaction volumes were calculated. Corresponding values of +5.4 (CML) and +9.9 cm³/mol (GOLD) indicate a suppressive effect of pressure on the formation of these glycation compounds. Above this, several other known end-products, namely N(6)-glycoloyllysine (GALA), N(6)-[2-[(5-amino-5-carboxypentyl)amino]-2-oxoethyl]lysine (GOLA), and reaction intermediates, were qualitatively monitored and proofed a similar behavior compared to the GOLD formation pathway. The reasons for the influence of pressure are discussed in the context of the reaction mechanism.





Similar content being viewed by others
References
Hite BH (1899) The effect of pressure in the preservation of milk. West Virginia Univ Agr Expt Sta Morgantown Bull 58::15–35
Hendrickx M, Knorr D (2001) Ultra high pressure treatments of foods. Kluwer Academic/Plenum Publishers, New York
Cheftel JC (1995) Review: high-pressure, microbial inactivation and food preservation. Food Sci Technol Int 1:75–90
Margosch D, Ehrmann MA, Gänzle MG, Vogel RE (2004) Comparison of pressure and heat resistance of Clostridium botulinum and other endospores in mashed carrots. J Food Protect 67:2530–2537
Tauscher B (1995) Pasteurization of food by hydrostatic high pressure: chemical aspects. Z Lebensm Unters Forsch 200:3–13
Tamaoka T, Itoh N, Hayashi R (1991) High pressure effect on the Maillard reaction. Agric Biol Chem 55:2071–2074
Isaacs N, Coulson M (1996) Effect of pressure on processes modelling the Maillard reaction. J Phys Org Chem 9:639–644
Hill VM, Ledward DA, Ames JM (1996) Influence of high hydrostatic pressure and pH on the rate of Maillard browning on a glucose-lysine system. J Agric Food Chem 44:594–598
Schwarzenbolz U, Klostermeyer H, Henle T (2000) Maillard-type reactions under pressure: formation of pentosidine. Eur Food Res Technol 211:208–210
Schwarzenbolz U, Klostermeyer H, Henle T (2002) Maillard reaction under high hydrostatic pressure: studies on the formation of protein-bound amino acid derivatives. In: Horiuchi S, Taniguchi N, Hayase F, Kurata T, Osawa T (eds) The Maillard reaction in food chemistry and medical science: Update for the postgenomic era, 7th International Symposium on the Maillard Reaction. Elsevier Science, Amsterdam, pp 223–227t;/bib>
Kebede BT, Grauwet T, Tabilo-Munizaga G, Palmers S, Vervoort L, Hendrickx M, Van Loey A (2013) Headspace components that discriminate between thermal and high pressure high temperature treated green vegetables: identification and linkage to possible process-induced chemical changes. Food Chem 213:1603–1613
Schwarzenbolz U, Henle T (2010) Non-enzymatic modifications of proteins under high-pressure treatment. High Pressure Res 30:458–465
Alt N, Schieberle P (2005) Model studies on the influence of high hydrostatic pressure on the formation of glycated arginine modifications at elevated temperatures. J Agric Food Chem 53:5789–5797
Ahmed MU, Thorpe SR, Baynes JW (1986) Identification of N ε-(carboxymethyl)lysine as a degradation product of fructoselysine in glycated protein. J Biol Chem 261:4889–4894
Glomb MA, Monnier VM (1995) Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem 270:10017–10026
Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW (1998) Role of the Maillard reaction in aging of tissue proteins: advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J Biol Chem 273:18714–18719
Wells-Knecht KJ, Brinkmann E, Baynes JW (1995) Characterization of an Imidazolium salt formed from glyoxal and N-hippuryllysine: a model for maillard reaction crosslinks in proteins. J Org Chem 60: 6246–6247
Glomb MA, Pfahler C (2001) Amides are novel protein modifications formed by physiological sugars. J Biol Chem 276:41638–41647
Wells-Knecht KJ, Brinkmann E, Wells-Knecht MC, Litchfield JE, Ahmed MU, Reddy S, Zyzak DV, Thorpe SR, Baynes JW (1996) New biomarkers of Maillard reaction damage to proteins. Nephrol Dial Transpl 11(Suppl 5): 41–47
Cho S, Roman G, Yeboah F, Konishi Y (2007) The road to advanced glycation end products: a mechanistic perspective. Curr Med Chem 14:1653–1671
Asano T, Le Noble WJ (1978) Activation and reaction volumes in solution. Chem Rev 78:407–489
Baisier WM, Labuza TP (1992) Maillard Browning kinetics in a liquid model system. J Agric Food Chem 40:707–713
Cerrutti P, Resnik SL, Seldes A, Ferro-Fontan C (1985) Kinetics of deteriorative reactions in model food systems of high water activity: glucose loss, 5-hydroxymethylfurfural accumulation and fluorescence development due to nonenzymatic browning. J Food Sci 50:627–630
Saraiva M, Borges C, Florêncio H (2006) Reactions of a modified lysine with aldehydic and diketonic dicarbonyl compounds: an electrospray mass spectrometry structure/activity study. J Mass Spectrom 41:216–228
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Schwarzenbolz, U., Förster, A. & Henle, T. Influence of high hydrostatic pressure on the reaction between glyoxal and lysine residues. Eur Food Res Technol 243, 1355–1361 (2017). https://doi.org/10.1007/s00217-017-2846-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2846-x