Abstract
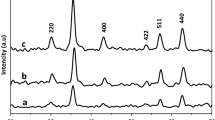
An efficient immobilization of Alcalase 2.4L alkaline protease has been developed by using chitosan-coated magnetic nanoparticles as support via glutaraldehyde cross-linking reaction. The Fe3O4 nanoparticles, Fe3O4-chitosan, and immobilized Alcalase 2.4L alkaline protease were characterized by X-ray diffraction, transmission electron microscope, Fourier transform infrared spectroscopy, electron spin resonance, and vibrating sample magnetometry. Results showed that the binding of chitosan and Alcalase 2.4L alkaline protease on Fe3O4 through cross-linking was successful. In addition, the Alcalase 2.4L alkaline protease immobilized with chitosan-coated magnetic nanoparticles enhanced the activity, the optimum reaction temperature and pH value for the immobilized enzyme were 55 °C and 10, respectively, compared with the free enzyme, and the optimal temperature and pH profile range were considerably broadened. Similarly, the thermal stability was enhanced by immobilization, and the kinetic parameters of free and immobilized Alcalase 2.4L alkaline protease were determined. Then, from our hydrolysis experiments, we found that immobilized Alcalase 2.4L alkaline protease uses Fe3O4-chitosan had a greatest hydrolytic activity, and the DH of soy protein isolate (SPI) can reach to 18.38 %, against 17.50 % with the free enzyme after 140 min. Furthermore, the immobilized Alcalase 2.4L alkaline protease could maintain about 86 % of its original activity after ten consecutive operations. Thus, Fe3O4-chitosan immobilized Alcalase 2.4L alkaline protease a good candidate for the continuous hydrolysis of SPI.










Similar content being viewed by others
References
Sun XD (2011) Enzymatic hydrolysis of soy proteins and the hydrolysates utilisation. Int J Food Sci Tech 46:2447–2459
Tavano OL (2013) Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal B Enzym 90:1–11
Zeng M, Adhikari B, He Z, Qin F, Huang X, Chen J (2013) Improving the foaming properties of soy protein isolate through partial enzymatic hydrolysis. Dry Technol 31:1545–1552
Zhao X, Hou Y (2009) Limited hydrolysis of soybean protein concentrate and isolate with two proteases and the impact on emulsifying activity index of hydrolysates. Afr J Biotechnol 8:3314–3319
Chen L, Chen J, Ren J, Zhao M (2011) Modifications of soy protein isolates using combined extrusion pre-treatment and controlled enzymatic hydrolysis for improved emulsifying properties. Food Hydrocoll 25:887–897
Lamsal BP, Jung S, Johnson LA (2007) Rheological properties of soy protein hydrolysates obtained from limited enzymatic hydrolysis. Lwt-Food Sci Technol 40:1215–1223
Su R, Zhang W, Zhu M, Xu S, Yang M, Li D (2013) Alkaline protease immobilized on graphene oxide: highly efficient catalysts for the proteolysis of waste-activated sludge. Pol J Environ Stud 22:885–891
Li Y, Shoemaker CF, Ma J, Luo C, Zhong F (2009) Effects of alcalase/protease N treatments on rice starch isolation and their effects on its properties. Food Chem 114:821–828
Balti R, Bougatef A, El Hadj Ali N, Ktari N, Jellouli K, Nedjar-Arroume N, Dhulster P, Nasri M (2011) Comparative study on biochemical properties and antioxidative activity of cuttlefish (Sepia officinalis) protein hydrolysates produced by alcalase and Bacillus licheniformis NH1 proteases. J Amino Acids 2011:107179-107179
Chen ST, Chen SY, Hsiao SC, Wang KT (1991) Application of industrial protease “Alcalase” in peptide synthesis. Biomed Biochim Acta 50:S181–S186
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym Microb Tech 40:1451–1463
Tang Z-X, Qian J-Q, Shi L-E (2006) Characterizations of immobilized neutral proteinase on chitosan nano-particles. Process Biochem 41:1193–1197
Kumar AG, Swarnalatha S, Kamatchi P, Sekaran G (2009) Immobilization of high catalytic acid protease on functionalized mesoporous activated carbon particles. Biochem Eng J 43:185–190
Rocha C, Gonçalves MP, Teixeira JA (2011) Immobilization of trypsin on spent grains for whey protein hydrolysis. Process Biochem 46:505–511
Sahin F, Demirel G, Tumturk H (2005) A novel matrix for the immobilization of acetylcholinesterase. Int J Biol Macromol 37:148–153
Y-h Ma, Chen G, Zhao J (2013) Preparation and characterization of chitosan coated magnetic nanoparticles and their bsa adsorption properties. Acta Polym Sin 1:1369–1375
Mohapatra S, Mallick SK, Maiti TK, Ghosh SK, Pramanik P (2007) Synthesis of highly stable folic acid conjugated magnetite nanoparticles for targeting cancer cells. Nanotechnology 18:1–9
Adler-Nissen J (1979) Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem 27:1256–1262
Liu Y, Jia S, Wu Q, Ran J, Zhang W, Wu S (2011) Studies of Fe3O4-chitosan nanoparticles prepared by co-precipitation under the magnetic field for lipase immobilization. Catal Commun 12:717–720
Bayramoğlu G, Yılmaz M, Şenel AÜ, Arıca MY (2008) Preparation of nanofibrous polymer grafted magnetic poly(GMA-MMA)-g-MAA beads for immobilization of trypsin via adsorption. Biochem Eng J 40:262–274
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Cheml Soc Rev 42:6223–6235
Mendes AA, Castro HF, Rodrigues DS, Adriano WS, Tardioli PW, Mammarella EJ, Giordano RC, Giordano RLC (2011) Multipoint covalent immobilization of lipase on chitosan hybrid hydrogels: influence of the polyelectrolyte complex type and chemical modification on the catalytic properties of the biocatalysts. J Ind Microbiol Biotechnol 38:1055–1066
Mendes AA, Giordano RC, Giordano RLC, Castro HF (2011) Immobilization and stabilization of microbial lipases by multipoint covalent attachment on aldehyde-resin affinity: application of the biocatalysts in biodiesel synthesis. J Mol Catal B Enzym 68:109–111
Vieira DC, Lima LN, Mendes AA, Adriano WS, Giordano RC, Giordano RLC, Tardioli PW (2013) Hydrolysis of lactose in whole milk catalyzed by β-galactosidase from Kluyveromyces fragilis immobilized on chitosan-based matrix. Biochem Eng J 81:54–64
Tsou MJ, Kao FJ, Lu HC, Kao HC, Chiang WD (2013) Purification and identification of lipolysis-stimulating peptides derived from enzymatic hydrolysis of soy protein. Food Chem 138:1454–1460
Kumar S, Jana AK, Dhamija I, Singla Y, Maiti M (2013) Preparation, characterization and targeted delivery of serratiopeptidase immobilized on amino-functionalized magnetic nanoparticles. Eur J Pharm Biopharm 85:413–426
Li D, Teoh WY, Gooding JJ, Selomulya C, Amal R (2010) Functionalization strategies for protease immobilization on magnetic nanoparticles. Adv Funct Mater 20:1767–1777
Zhuang H, Dong S, Zhang T, Tang N, Xu N, Sun B, Liu J, Zhang M, Yuan Y (2014) Study on alkaline protease immobilized on mesoporous materials. Asian J Chem 26:1139–1144
Benkhelifa H, Bengoa C, Larre C, Guibal E, Popineau Y, Legrand J (2005) Casein hydrolysis by immobilized enzymes in a torus reactor. Process Biochem 40:461–467
Acknowledgments
This study was supported by the National High Technology Research and Development Program of China (863 Program: 2013AA102104-1, 2013AA102104-4), the Synergetic Innovation Center of Food Safety and Nutrition. We gratefully acknowledge the financial support provided by National Soybean Industrial Technology System (Grant No. CARS-04-PS25) for the research.
Conflict of interest
The authors declare that they have no competing interests.
Compliance with Ethics Requirements
We declare that we do not have any commercial or associative interest and the research has fully complied with research ethics.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Sn., Zhang, Cr., Qi, Bk. et al. Immobilized alcalase alkaline protease on the magnetic chitosan nanoparticles used for soy protein isolate hydrolysis. Eur Food Res Technol 239, 1051–1059 (2014). https://doi.org/10.1007/s00217-014-2301-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2301-1