Abstract
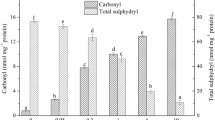
In this study, shrimp tropomyosin was subjected to malonaldehyde (MDA)-induced oxidative stress in aqueous situation. The in vivo cross-linking of tropomyosin was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the structural changes were investigated by differential scanning calorimetry, circular dichroism, and Fourier transform infrared spectroscopy (FTIR). The location of the resulting protein carbonyls was determined by mass spectrometry. The SDS-PAGE of the cross-linked tropomyosin showed four new bands corresponding to two, three, four, and five-time molecular weights of tropomyosin. The conformational structures were partly destroyed because of the thermal denaturation at higher temperatures. The α-helix content increased, and new chemical bonds were formed by the MDA modification. These physiochemical properties were confirmed by the identification of amino acid side-chain modifications by using liquid chromatography–tandem mass spectrometry. Lysine (Lys), glutamine (Gln), and asparagine (Asn) residues in tropomyosin were modified at four lysine (Lys-76, 168, 189, and 233), five glutamine (Gln-61, 70, 118, 147, and 247), and four asparagine (Asn-17, 107, 203, and 215) sites. Apart from these MDA modification sites, oxidized methionine was observed by data search. These results indicate that some active food ingredients, such as MDA, can react with the side chains of amino acids resulting in structural changes in the protein.







Similar content being viewed by others
References
Guangming L, Minjie C, Qiufeng C, Haiwang S, Weiwei R, Wenjin S (2012) Current status and future perspectives in research on seafood allergens. J Chin Inst Food Sci Tech 12(5):1–9
Martínez A, Portero-Otin M, Pamplona R, Ferrer I (2010) Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol 20(2):281–297
Sarmadi BH, Ismail A (2010) Antioxidative peptides from food proteins: a review. Peptides 31(10):1949–1956
Jones LM, Sperry JB, Carroll JA, Gross ML (2011) Fast photochemical oxidation of proteins for epitope mapping. Anal Chem 83(20):7657–7661
Sharma J, Yeh HC, Yoo H, Werner JH, Martinez JS (2011) Silver nanocluster aptamers: in situ generation of intrinsically fluorescent recognition ligands for protein detection. Chem Commun 47(8):2294–2296
Zhao J, Chen J, Zhu H, Xiong YL (2012) Mass spectrometric evidence of malonaldehyde and 4-hydroxynonenal adductions to radical-scavenging soy peptides. J Agric Food Chem 60(38):9727–9736
Adams A, De Kimpe N, van Boekel MAJS (2008) Modification of casein by the lipid oxidation product malondialdehyde. J Agric Food Chem 56(5):1713–1719
Guo J, Prokai-Tatrai K, Nguyen V, Rauniyar N, Ughy B, Prokai L (2011) Protein targets for carbonylation by 4-hydroxy-2-nonenal in rat liver mitochondria. J Proteomics 74(11):2370–2379
Sayre LM, Lin D, Yuan Q, Zhu X, Tang X (2006) Protein adducts generated from products of lipid oxidation: focus on HNE and ONE. Drug Metab Rev 38(4):651–675
Hu M, McClements DJ, Decker EA (2003) Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J Agric Food Chem 51(6):1696–1700
Zhenxing L, Hong L, Limin C, Jamil K (2007) The influence of gamma irradiation on the allergenicity of shrimp (Penaeus vannamei). J Food Eng 79(3):945–949
Byun MW, Kim JH, Lee JW, Park JW, Hong CS, Kang IJ (2000) Effects of gamma radiation on the conformational and antigenic properties of a heat-stable major allergen in brown shrimp. J Food Prot 63(7):940–944
Lee JW, Seo JH, Kim JH, Lee SY, Byun MW (2007) Comparison of the changes of the antigenicities of a hen’s egg albumin by a gamma and an electron beam irradiation. Radiat Phys Chem 76(5):879–885
Chu KH, Wong SH, Leung PSC (2000) Tropomyosin is the major mollusk allergen: reverse transcriptase polymerase chain reaction, expression and IgE reactivity. Mar Biotechnol 2(5):499–509
Rajagopal D, Ganesh KA, Subba Rao PV (2000) Modulation of allergen-specific immune responses to the major shrimp allergen, tropomyosin, by specific targeting to scavenger receptors on macrophages. Int Arch Allergy Immunol 121(4):308–316
Ayuso R, Lehrer SB, Reese G (2002) Identification of continuous, allergenic regions of the major shrimp allergen pen a 1 (tropomyosin). Int Arch Allergy Immunol 127(1):27–37
Adams A, De Kimpe N, van Boekel MAJS (2008) Modification of casein by the lipid oxidation product malondialdehyde. J Agric Food Chem 56(5):1713–1719
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem 72(1–2):248–254
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Jansson H, Swenson J (2010) The protein glass transition as measured by dielectric spectroscopy and differential scanning calorimetry. BBA-Proteins Proteomics 1804(1):20–26
Kelly SM, Jess TJ, Price NC (2005) How to study proteins by circular dichroism. BBA-Proteins Proteomics 1751(2):119–139
Toyran N, Zorlu F, Dönmez G, Öğe K, Severcan F (2004) Chronic hypoperfusion alters the content and structure of proteins and lipids of rat brain homogenates: a Fourier transform infrared spectroscopy study. Eur Biophys J 33(6):549–554
Martínez A, Portero-Otin M, Pamplona R, Ferrer I (2010) Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol 20(2):281–297
Liu G, Xiong YL, Butterfield DA (2000) Chemical, physical, and gel-forming properties of oxidized myofibrils and whey-and soy-protein isolates. J Food Sci 65(5):811–818
Sarker M, Rose J, McDonald M, Morrow MR, Booth V (2010) Modifications to surfactant protein B structure and lipid interactions under respiratory distress conditions: consequences of tryptophan oxidation. Biochem 50(1):25–36
Narayan M, Welker E, Wedemeyer WJ, Scheraga HA (2000) Oxidative folding of proteins. Accounts Chem Res 33(11):805–812
McClain DL, Gurnon DG, Oakley MG (2002) Importance of potential interhelical salt-bridges involving interior residues for coiled-coil stability and quaternary structure. J Mol Biol 324(2):257–270
Davies KJA (2001) Degradation of oxidized proteins by the 20S proteasome. Biochimie 83(3):301–310
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329(1):23–38
Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villén J, Li J, Gygi SP (2004) Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci USA 101(33):12130–12135
Butterfield DA, Lauderback CM (2002) Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic Bio Med 32(11):1050–1060
Acknowledgments
The authors thank Dr. Fali Bai from Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences for helping with mass spectrometry analysis. Thanks are due to Ravindra Pawar who helped with the linguistic revision of the manuscript. This work was supported by NSFC (31371730), National Science & Technology Pillar Program (2012BAD28B05) and Program for Changjiang Scholars and Innovative Research Team in University.
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, L., Lin, H., Li, Z. et al. Identification of oxidative modification of shrimp (Metapenaeus ensis) tropomyosin induced by malonaldehyde. Eur Food Res Technol 239, 847–855 (2014). https://doi.org/10.1007/s00217-014-2281-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2281-1