Abstract
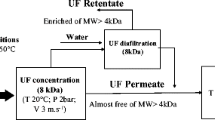
In order to utilize yellowfin sole ( Limanda aspera) frame protein (YFP), which is normally discarded as industrial waste in the process of fish manufacture, yellowfin sole frame protein hydrolysates (YFPHs) were fractionated using an ultrafiltration (UF) membrane system following hydrolysis with pepsin and mackerel intestines crude enzyme (MICE). The YFPHs were separated into five major types, YFPH-I (30–10 kDa), YFPH-II (10–5 kDa), YFPH-III (5–3 kDa), YFPH-IV (3–1 kDa), and YFPH-V (below 1 kDa) by using UF membranes with molecular weight cut-offs of 30, 10, 5, 3, and 1 kDa, respectively. The antioxidative activity of the YFPHs was investigated and compared with that of a natural antioxidant, α-tocopherol, used as a reference. Furthermore, the fraction showing strong antioxidative activity was isolated from the YFPHs using consecutive chromatographic methods on an SP-Sephadex C-25 column, on a Sephadex G-75 column, and high-performance liquid chromatography (HPLC) on an octadecylsilane column. The molecular mass of the antioxidant was identified as 13 kDa using HPLC on a gel permeation chromatography (GPC) column, and the antioxidative peptide was composed of 10 N-terminal amino acid residues, RPDFDLEPPY.





Similar content being viewed by others
References
Maillard MG, Soum MH, Meydani SN, Berset C (1996) Food Sci Technol 29:238–244
Wanita A, Lorenz K (1996) J Food Process Preserv 20:417–429
Hettiarachchy NS, Glenn KC, Gnanasambandam R, Johnson MG (1996) J Food Sci 61:516–519
Frlich I, Riederer P (1995) Drug Res 45:443–449
Yamaguchi NS, Naito Y, Yokoo Y, Fujimaki M (1980) J Jpn Soc Food Sci Technol 27:56–59
Pratt DE (1972) J Food Sci 37:322–323
Yukami S (1972) Agric Biol Chem 36:871–874
Rhee KS, Ziprin YA, Rhee KC (1979) J Food Sci 44:1132–1135
Iwami K, Hattori M, Ibuki F (1987) J Agric Food Chem 35:628–631
Chavan UD, Amarowicz R, Shahidi F (1999) J Food Lipids 9:1-11
Shahidi F, Amarowicz R, He Y, Wettasinghe M (1997) J Food Lipids 7:75–86
Wang JY, Fujimoto K, Miyazawa T, Endo Y (1991) J Agric Food Chem 39:351–355
Park PJ, Jung WK, Nam KS, Shahidi F, Kim SK (2001) J Am Oil Chem Soc 78:651–656
Kim SK, Kim YT, Byun HG, Nam KS, Joo DS, Shahidi F (2001) J Agric Food Chem 49:1984–1989
Park PJ, Je JY, Kim SK (2003) J Agric Food Chem 51:4624–4627
Podsedek, A, sosnowska D, Anders B (2003) Eur Food Res Technol 217:296–300
Carlsen CU, Rasmussen KT, Kjeldsen KK, Westergaard P, Skibsted LH (2003) Eur Food Res Technol 217:195–200
Gopala KAG, Prabhakar JV (1994) J Am Oil Chem Soc 71:645–647
Kim SK, Park PJ, Byun HG, Je JY, Moon SH (2003) J Food Biochem 27:255-266
Osawa T, Namiki M (1985) J Agric Food Chem 33:777–780
Ohkawa H, Ohishi N, Yagi K (1979) Anal Biochem 95:351–358
Mitsuda H, Yasumoto K, Iwami K (1966) Eiyo to Shokuryo 19:210–214
Kim SK, Jeon YJ, Byun HG, Kim YT, Lee CK (1997) Fish Sci 63:421–528
Receca BD, Pena-Vera MT, Deaz-Castaneda M (1991) J Food Sci 56:309–314
Nair AL, Gopakumar K (1982) Fish Technol 19:101–103
Bishov SJ, Henick AS (1975) J Food Sci 40:345–348
Kim SK, Kim YT, Byun HG, Nam KS, Joo DS, Shahidi F (2001) J Agric Food Chem 49:1984–1989
Hatate H, Nagata Y, Kochi M (1990) Yukagaku 39:42–46
Chen HM, Muramoto K, Yamauchi F, Nokihara K (1996) J Agric Food Chem 44:2619–2623
Riisom T, Sims RJ, Fiorti JA (1980) J Am Oil Chem Soc 57:351–359
Taylor MJ, Richardson T (1980) J Food Sci 45:1223–1227
Yee JJ, Shipe WF, Kinsella JE (1980) J Food Sci 45:1082–1083
Kawashima K, Itoh H, Miyoshi M, Chibata I (1979) Chem Pharm Bull 40:1912–1916
Bishov SJ, Henick AS (1972) Chem Pharm Bull 37:873–875
Acknowledgments
This work was supported by the MOST, Busan Metropolitan City, and Daerim Co. in Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jun, SY., Park, PJ., Jung, WK. et al. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole ( Limanda aspera) frame protein. Eur Food Res Technol 219, 20–26 (2004). https://doi.org/10.1007/s00217-004-0882-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-004-0882-9