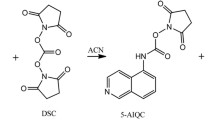
Abstract
Amino acids, neurotransmitters, purines, and pyrimidines are bioactive molecules that play fundamental roles in maintaining various physiological functions. Their metabolism is closely related to the health, growth, development, reproduction, and homeostasis of organisms. Most recently, comprehensive measurements of these metabolites have shown their potential as innovative approaches in disease surveillance or drug intervention. However, simultaneous measurement of these metabolites presents great difficulties. Here, we report a novel quantitative method that uses hydrophilic interaction ultra-high-performance liquid chromatography–tandem mass spectrometry (HILIC-UPLC–MS/MS), which is highly selective, high throughput, and exhibits better chromatographic behavior than existing methods. The developed method enabled the rapid quantification of 37 metabolites, spanning amino acids, neurotransmitters, purines, and pyrimidines pathways, within 6.5 min. The compounds were separated on an ACQUITY UPLC® BEH Amide column. Serum and brain homogenate were extracted by protein precipitation. The intra- and interday precision of all of the analytes was less than 11.34 %, and the accuracy was between −11.74 and 11.51 % for all quality control (QC) levels. The extraction recoveries of serum ranged from 84.58 % to 116.43 % and those of brain samples from 80.80 % to 119.39 %, while the RSD was 14.61 % or less for all recoveries. This method was used to successfully characterize alterations in the rat brain and, in particular, their dynamics in serum. The following study was performed to simultaneously test global changes of these metabolites in a serotonin antagonist p-chlorophenylalanine (PCPA)-induced anxiety and insomnia rat model to understand the effect and mechanism of PCPA. Taken together, these results show that the method is able to simultaneously monitor a large panel of metabolites and that this protocol may represent a metabolomic method to diagnose toxicological and pathophysiological states.

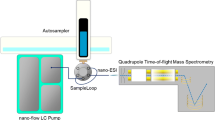
Overview of experimental workflow





Similar content being viewed by others
References
Brosnan JT. Amino acids, then and now – a reflection on Sir Hans Krebs’ contribution to nitrogen metabolism. IUBMB Life. 2001;52:265–70.
Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17.
Shibata H, Ochiai H, Sawa Y, Miyoshi S. Localization of carbamoylphosphate synthetase and aspartate carbamoyltransferase in chloroplasts. Plant Physiol. 1986;80:126–9.
Chalcraft KR, Britz-McKibbin P. Newborn screening of inborn errors of metabolism by capillary electrophoresis-electrospray ionization-mass spectrometry: a second-tier method with improved specificity and sensitivity. Anal Chem. 2009;81:307–14.
Kuhara T. Gas chromatographic-mass spectrometric urinary metabolome analysis to study mutations of inborn errors of metabolism. Mass Spectrom Rev. 2005;24:814–27.
Fleming RA, Milano G, Thyss A, Etienne MC, Renee N, Schneider M, et al. Correlation between dihydropyrimidine dehydrogenase activity in peripheral mononuclear cells and systemic clearance of fluorouracil in cancer patients. Cancer Res. 1992;52:2899–902.
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77.
Proctor DT, Coulson EJ, Dodd PR. Post-synaptic scaffolding protein interactions with glutamate receptors in synaptic dysfunction and Alzheimer's disease. Prog Neurobiol. 2011;93:509–21.
Gottwald MD, Aminoff MJ. Therapies for dopaminergic-induced dyskinesias in Parkinson disease. Ann Neurol. 2011;69:919–27.
Ghia JE, Li N, Wang H, Collins M, Deng Y, EI-Sharkawy RT, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–60.
Magro F, Vieira-Coelho MA, Fraga S, Serrao MP, Veloso FT, Ribeiro T, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47:216–24.
Zheng X, Kang A, Dai C, Liang Y, Xie T, Xie L, et al. Quantitative analysis of neurochemical panel in rat brain and plasma by liquid-chromatography-tandem mass spectrometry. Anal Chem. 2012;84:10044–51.
Brauer MJ, Yuan J, Bennett BD, Lu W, Kimball E, Botstein D, et al. Conservation of the metabolomic response to starvation across two divergent microbes. Proc Natl Acad Sci U S A. 2006;103:19302–7.
Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214.
Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM. Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal Chem. 2006;78:4430–42.
Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–67.
Ru W, Li G, Seymour AB. High-throughput and multiplexed LC/MS/MRM method for targeted metabolomics. Anal Chem. 2010;82:5527–33.
Soga T, Ohashi Y, Ueno Y, Naraoka H, Tomita M, Nishioka T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J Proteome Res. 2003;2:488–94.
Nordstrom A, Want E, Northen T, Lehtio J, Siuzdak G. Multiple ionization mass spectrometry strategy used to reveal the complexity of metabolomics. Anal Chem. 2008;80:421–9.
Patterson AD, Li H, Eichler GS, Kristopher KW, Weinstein Jr JN, Fornace AJ, et al. UPLC-ESI-TOFMS-based metabolomics and gene expression dynamics inspector self-organizing metabolomic maps as tools for understanding the cellular response to ionizing radiation. Anal Chem. 2008;80:665–74.
Kaspar H, Dettmer K, Gronwald W, Oefner PJ. Advances in amino acid analysis. Anal Bioanal Chem. 2009;393:445–52.
Petritis KNC, Elfakir PC, Dreux M. Automatic recording apparatus for use in the chromatography of amino acids. J Chromatogr A. 1999;833:147–55.
Deyl Z, Hyanek J, Horakova M. Profiling of amino acids in body fluids and tissues by means of liquid chromatography. J Chromatogr. 1986;379:177–250.
Sarwar G, Botting HG. Evaluation of liquid chromatographic analysis of nutritionally important amino acids in food and physiological samples. J Chromatogr. 1993;615:1–22.
Moore S, Spackamn DH, Stein WH. Automatic recording apparatus for use in the chromatography of amino acids. Fed Proc. 1958;17:1107–15.
Langrock T, Czihal P, Hoffmann R. Amino acid analysis by hydrophilic interaction chromatography coupled on-line to electrospray ionization mass spectrometry. Amino Acids. 2006;30:291–7.
Linden JC, Lawhead CL. Liquid chromatography of saccharides. J Chromatogr A. 1975;105:125–33.
Alpert AJ. Hydrophiliic-interaction chromatography ofr the separation of peptides, nucleic acids and other polar compounds. J Chromatogr. 1990;499:177–96.
Churms SC. Recent progress in carbohydrate sepatation by high-performance liquid chromatography based on hydrophilic interaction. J Chromatogr A. 1996;720:75–91.
Padivitage NL, Dissanayake MK, Armstrong DW. Separation of nucleotides by hydrophilic interaction chromatography using the FRULIC-N column. Anal Bioanal Chem. 2013;405:8837–48.
Hao Z, Lu CY, Xiao B, Weng N, Parker B, Knapp M, et al. Separation of amino acids, peptides and corresponding Amadori compounds on a silica column at elevated temperature. J Chromatogr A. 2007;1147:165–71.
Gritti F, dos Santos Pereira A, Sandra P, Guiochon G. Efficiency of the same neat silica column in hydrophilic interaction chromatography and per aqueous liquid chromatography. J Chromatogr A. 2010;1217:683–8.
Schlichtherle-Cerny H, Affolter M, Cerny C. Hydrophilic interaction liquid chromatography coupled to electrospray mass spectrometry of small polar compounds in food analysis. Anal Chem. 2003;75:2349–54.
Jiang H, Jiang J, Hu P, Hu Y. Measurement of endogenous uracil and dihydrouracil in plasma and urine of normal subjects by liquid chromatography–tandem mass spectrometry. J Chromatogr B. 2002;769:169–76.
Ni M, Duley J, George R, Charles B, Shannon C, McGeary R, et al. Simultaneous determination of thymine and its sequential catabolites dihydrothymine and β-ureidoisobutyrate in human plasma and urine using liquid chromatography-tandem mass spectrometry with pharmacokinetic application. J Pharm Biomed. 2013;78–79:129–35.
Bourgogne E, Mathy FX, Boucaut D, Boekens H, Laprevote O. Simultaneous quantitation of histamine and its major metabolite 1-methylhistamine in brain dialysates by using precolumn derivatization prior to HILIC-MS/MS analysis. Anal Bioanal Chem. 2012;402:449–59.
Buck K, Voehringer P, Ferger B. Rapid analysis of GABA and glutamate in microdialysis samples using high performance liquid chromatography and tandem mass spectrometry. J Neurosci Methods. 2009;182:78–84.
Danaceau JP, Chambers EE, Fountain KJ. Hydrophilic interaction chromatography (HILIC) for LC–MS/MS analysis of monoamine neurotransmitters. Bioanalysis. 2012;4:783–94.
Zhou G, Pang H, Tang Y, Yao X, Mo X, Zhu S, et al. Hydrophilic interaction ultra-performance liquid chromatography coupled with triple-quadrupole tandem mass spectrometry for highly rapid and sensitive analysis of underivatized amino acids in functional foods. Amino Acids. 2013;44:1293–305.
Sriboonvorakul N, Leepipatpiboon N, Dondorp AM, Pouplin T, White NJ, Tarning J, et al. Liquid chromatographic-mass spectrometric method for simultaneous determination of small organic acids potentially contributing to acidosis in severe malaria. J Chromatogr B. 2013;941:116–22.
Guadagni R, Miraglia N, Simonelli A, Silvestre A, Lamberti M, Feola D, et al. Solid-phase microextraction-gas chromatography-mass spectrometry method validation for the determination of endogenous substances: urinary hexanal and heptanal as lung tumor biomarkers. Anal Chim Acta. 2011;701:29–36.
van de Merbel NC. Quantitative determination of endogenous compounds in biological samples using chromatographic techniques. TrAC Trends Anal Chem. 2008;27:924–33.
de-Miguel FF, Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cell Mol Neurobiol. 2005;25:297–312.
Lepetit P, Touret M, Grange E, Gay N, Bobillier P. Inhibition of methionine incorporation into brain proteins after the systemic administration of p-chlorophenylalanine and L-5-hydroxytryptophan. Eur J Pharmacol. 1991;209:207–12.
Lepetit P, Touret M, Grange E, Gay E, Bobillier P. Decreased protein synthesis in hypothalamic nuclei following L-5-hydroxytryptophan in intact and p-chlorophenylalanine-pretreated rats. Neurosci Lett. 1991;209:218–20.
Koe BK, Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1996;154:499–516.
Romaniuk A, Strzelczuk M, Wieczorek M. Serotonin depletion with p-chlorophenylalanine in the cat: effects on carbachol-induced defensive behavior and regional brain amine content. Acta Neurobiol Exp. 1989;49:130–40.
Fonteh AN, Harrington RJ, Tsai A, Liao P, Harrington MG. Free amino acid and dipeptide changes in the body fluids from Alzheimer's disease subjects. Amino Acids. 2007;32:213–24.
Broadley KJ. The vascular effects of trace amines and amphetamines. Pharmacol Ther. 2010;125:363–75.
Lindemann L, Hoener MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol. 2005;26:274–81.
Sjoberg S, Eriksson M, Nordin C. L-Thyroxine treatment and neurotransmitter levels in the cerebrospinal fluid of hypothyroid patients: a pilot study. Eur J Endocrinol. 1998;139:493–7.
Aprison MH, Werman R. The distribution of glycine in cat spinal cord and roots. Life Sci. 1965;21:2075–83.
de Koning TJ, Snell K, Duran M, Berger R, Poll-The BT, Surtees R. L-serine in disease and development. Biochem J. 2003;371:653–61.
Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54.
Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc RJ, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–68.
Scriver CR, McInnes RR, Mohyuddin F. Role of epithelial architecture and intracellular metabolism in proline uptake and transtubular reclamation in PRO/re mouse kidney. Proc Natl Acad Sci U S A. 1975;72:1431–5.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Ref. Nos. 81473319 and 81473540), Science and Technology Project of Guangdong Province (Nos. 2014A02020902, 2014A020221027, and 2015A030401031), and the Natural Science Foundation of Guangdong Province (No. 2015A030313123).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
All animal-use procedures were in accordance with the regulations for animal experimentation issued by the State Committee of Science and Technology of the People’s Republic of China. This study was approved by the Animal Ethics Committee of Guangzhou University of Chinese Medicine.
Additional information
Jiahui Chen and Waner Hou contributed equally to this work and should be considered co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 506 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Hou, W., Han, B. et al. Target-based metabolomics for the quantitative measurement of 37 pathway metabolites in rat brain and serum using hydrophilic interaction ultra-high-performance liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 408, 2527–2542 (2016). https://doi.org/10.1007/s00216-016-9352-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9352-z