Abstract
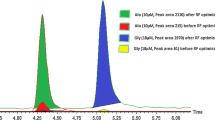
Trimethylamine N-oxide (TMAO) has attracted interest as circulating levels have reported prognostic value in patients with cardiovascular conditions, such as heart failure. With continual advances in accurate mass measurements, robust methods that can employ the capabilities of time of flight mass spectrometers would offer additional utility in the analysis of complex clinical samples. A Waters Acquity UPLC was coupled to a Waters Synapt G2-S high-resolution mass spectrometer. TMAO was measured in plasma by stable-isotope dilution-hydrophilic interaction liquid chromatography-time of flight mass spectrometry with multiple reaction monitoring (LC-ToF-MRM). Two transitions were monitored: m/z 76.1 to 58.066/59.073 and m/z 85.1 to 66.116/68.130. The method was assessed for linearity, lower limits of detection and quantitation, and reproducibility. A selected cohort of patients with systolic heart failure (SHF; n = 43) and healthy controls (n = 42) were measured to verify the assay is suitable for the analysis of clinical samples. Quantitative analysis of TMAO using LC-ToF-MRM enabled linearity to be established between 0.1 and 75 μmol/L, with a lower limit of detection of 0.05 μmol/L. Relative standard deviations reported an inter-day variation of ≤20.8 % and an intra-day variation of ≤11.4 % with an intra-study quality control variation of 2.7 %. Run times were 2.5 min. Clinical application of the method reported that TMAO in SHF was elevated compared to that of healthy controls (p < 0.0005). LC-ToF-MRM offers a highly selective method for accurate mass measurement of TMAO with rapid and reproducible results. Applicability of the method was shown in a selected cohort of patient samples.



Similar content being viewed by others
References
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63
Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL (2014) Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 64:1908–1914
Tang WHW, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL, Hazen SL (2015) Intestinal microbiota-dependent phosphatidylcholine metabolite, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail 21:91–96
Bae S, Ulrich CM, Neuhouser ML, Malysheva O, Bailey LB, Xiao L, Brown EC, Cushing-Haugen KL, Zheng Y, Cheng TY, Miller JW, Green R, Lane DS, Beresford SA, Caudill MA (2014) Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res 74:7442–7452
Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, McEntryre CJ, Elmslie JL, Atkinson W, Molyneux SL, Troughton RW, Frampton CM, Richards AM, Chambers ST (2014) Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One 9:e114969
Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL (2015) Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116:448–455
Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM (2015) The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep 10:326–338
Lenky C, McEntyre CJ, Lever M (2012) Measurement of marine osmolytes in mammalian serum by liquid chromatography-mass spectrometry. Anal Biochem 420:7–12
Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL (2014) Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem 44:35–40
Ocque AJ, Stubbs JR, Nolin TD (2015) Development and validation of a simple UHPLC-MS/MS method for the simultaneous determination of trimethylamine N-oxide, choline, and betaine in human plasma and urine. J Pharm Biomed Anal 109:128–135
Shushan B (2010) A review of clinical diagnostic applications of liquid chromatography-tandem mass spectrometry. Mass Spectrom Rev 29:930–944
Couchman L, Vincent RP, Ghataore L, Moniz CF, Taylor NF (2011) Challenges and benefits of endogenous steroid analysis by LC-MS/MS. Bioanalysis 3:2549–2572
Leung KS, Fong BM (2014) LC-MS/MS in the routine clinical laboratory: has its time come? Anal Bional Chem 406:2289–2301
Maurer HH (2007) Current role of liquid chromatography-mass spectrometry in clinical and forensic toxicology. Anal Bioanal Chem 388:1315–1325
Westberg EA, Singh R, Hedebrant U, Koukouves G, Souliotis VL, Farmer PB, Segerbäck D, Kyrtopoulos S, Tömqvist MA (2014) Adduct levels from benzo[a]pyrenediol epoxide: relative formation to histidine in serum albumin and to deoxyguanosine in DNA in vitro and in vivo in mice measured by LC/MS-MS methods. Toxicol Lett 26:28–36
Holman SW, Sims PF, Eyers CE (2012) The use of selected reaction monitoring in quantitative proteomics. Bioanalysis 4:1763–1786
Kushnir MM, Rockwood AL, Berqquist J (2010) Liquid chromatography-mass spectrometry applications in endocrinology. Mass Spectrom Rev 29:480–502
Vogeser M, Seger C (2010) Pitfalls associated with the use of liquid chromatography-tandem mass spectrometry in the clinical laboratory. Clin Chem 56:123–44
Couchman L, Benton CM, Moniz CF (2012) Variability in the analysis of 25-hydroxyvitamin D by liquid chromatography-tandem mass spectrometry: the devil is in the detail. Clin Chim Acta 413:1239–1243
Alelyunas YW, Wrona MD, Mortishire-Smith RJ, Tomczyk N, Rainville RD (2014) Quantitation by high resolution mass spectrometry: using target enhancement and Tof-MRM to achieve femtogram-level on-column sensitivity for quantitation of drugs in human plasma [application note]. Waters Corporation, Milford, MA, USA. http://www.waters.com/webassets/cms/library/docs/720005182en.pdf . Accessed 12 Oct 2015
Benton CM, Couchman L, Marsden JT, Rees DC, Moniz C, Lim CK (2013) Direct and simultaneous quantitation of 5-aminolaevulinic acid and porphobilinogen in human serum or plasma by hydrophilic interaction liquid chromatography-atmospheric pressure chemical ionization/tandem mass spectrometry. Biomed Chromatogr 27:267–272
US Food and Drug Administration/Centre for Drug Evaluation and Research (2001) Guidance for industry: bioanalytical method validation. FDA, Silver Spring
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75:3019–3030
NCCLS (1999) Evaluation of precision performance of clinical chemistry devices; approved guideline (EP5-A). NCCLS, Wayne
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL (2013) Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19:576–585
Petterys BJ, Graham KS, Parnás ML, Holt C, Frank EL (2012) Performance characteristics of an LC-MS/MS method for the determinations of plasma metanephrines. Clin Chim Acta 413:1459–1465
Dunand M, Donzelli M, Rickli A, Hysek CM, Liechti ME, Grouzmann E (2014) Analytical interference of 4-hydroxy-4-methoxymethamphetamine with the measurement of plasma free normetanephrine by ultra-high pressure liquid chromatography-tandem mass spectrometry. Clin Biochem 47:1121–1123
Gous T, Couchman L, Patel JP, Paradzai C, Arya R, Flanagan RJ (2014) Measurement of the direct oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in human plasma using turbulent flow liquid chromatography with high-resolution mass spectrometry. Ther Drug Monit 36:597–605
Minkler PE, Stoll MSK, Ingalls S, Kerner J, Hoppel CL (2015) Validated method for the quantification of free and total carnitine, butyrobetaine, and acylcarnitines in biological samples. Anal Chem. doi:10.1021/acs.analchem.5b02198
Gallien S, Duriez E, Crone C, Kellmann M, Moehring T, Domon B (2012) Targeted proteomic quantification on quadrupole-orbitrap mass spectrometer. Mol Cell Proteomics 11:1709–1723
Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ (2012) Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol Cell Proteomics 11:1475–1488
Gallien S, Bourmaud A, Kim SY, Domon B (2014) Technical considerations for large-scale parallel reaction monitoring analysis. J Proteome 100:147–159
Benton CM, Lim CK, Moniz C, Jones DJ (2012) Travelling wave ion mobility mass spectrometry of 5-aminolaevulinic acid, porphobilinogen and prophyrins. Rapid Commun Mass Spectrom 26:480–486
Daly CE, Ng LL, Hakimi A, Willingale R, Jones DJ (2014) Qualitative and quantitative characterization of plasma proteins when incorporating traveling wave ion mobility into a liquid chromatography-mass spectrometry workflow for biomarker discovery: use of product ion quantitation as an alternative data analysis tool for label free quantification. Anal Chem 86:1972–1979
Acknowledgments
This work was supported by the John and Lucille van Geest Foundation and the National Institute for Health Research Leicester Cardiovascular Biomedical Research Unit.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Liam M. Heaney and Donald J. L. Jones contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Standard addition experiments to assess recovery of trimethylamine N-oxide (TMAO) spiked into a pre-treated and untreated plasma matrix (PDF 7 kb)
Rights and permissions
About this article
Cite this article
Heaney, L.M., Jones, D.J.L., Mbasu, R.J. et al. High mass accuracy assay for trimethylamine N-oxide using stable-isotope dilution with liquid chromatography coupled to orthogonal acceleration time of flight mass spectrometry with multiple reaction monitoring. Anal Bioanal Chem 408, 797–804 (2016). https://doi.org/10.1007/s00216-015-9164-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9164-6